Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
A Review on Sublingual Dosage Form
Authors: Tejal Pawar , Raj Chitte , Sanjivani Bachhav, Deepali Deore, Prajakta Bachhav
DOI Link: https://doi.org/10.22214/ijraset.2025.66524
Certificate: View Certificate
Abstract
A sublingual tablet is a medication formulation that dissolves beneath the tongue, facilitating quick absorption of the active ingredients through the mucous membranes and into the bloodstream. Bypassing the liver metabolism and digestive tract, this technique can improve the effectiveness and speed of medication delivery. Sublingual medications are frequently used for drugs that need to start working quickly. Benefits of the sublingual route include a more consistent therapeutic impact and the avoidance of stomach upset. The salient features of the sublingual tablet include its ability to dissolve beneath the tongue, its speedy absorption and beginning of action, and its ability to prevent gastrointestinal irritation.
Introduction
I. INTRODUCTION
Sublingual dose forms entail putting medicine behind the tongue, where it dissolves and enters the bloodstream through the sublingual mucosa, which is extremely vascularized. Sublingual drug delivery emerged as a response to the need for a quick pharmacological effect. All age groups are susceptible to dysphasia, or difficulty swallowing, although it is especially common in the elderly, young children, mentally retarded, unwilling, nauseous, or dehydrated patients/dieters who have trouble swallowing various dosage forms. Medications administered sublingually enter the bloodstream directly through the ventral surface of the tongue and the floor of the mouth [1].
The drug is rapidly absorbed in the reticulated vein beneath the mouth mucosa and then enters the bloodstream through the brachiocephalic, internal jugular, and facial veins. a section of the mouth that is permeable. In the oral cavity, the sublingual, buccal (cheek), and palatal areas have the lowest levels of patency. Generally, the order is determined by the relative thickness and blood flow to each component. Sublingual: situated beneath the tongue .Sublingual dosage forms are any that are positioned under the tongue, dissolve, and enable the medication to be instantly absorbed through the sublingual mucosa.The dose form is not meant to be chewed or eaten. When a medication is administered sublingually, it is inserted beneath the tongue and immediately reaches the bloodstream through the floor of the mouth and the tongue's ventral surface. The drug's dissolved active components enter the reticulated vein and are quickly absorbed [2].
Sublingual Gland:The seromucous, polystomatic gland is called the glandula sublingualis. The sublingual gland, together with the submandibular and parotid, is the smallest and most pervasive of the three primary salivary glands in the oral cavity. Located beneath the mouth diaphragm, the sublingual gland produces roughly 3–5% of the salivary volume.They are situated anteriorly and superiorly to the submandibular gland, inferiorly and laterally to the tongue, and beneath the mucous membrane of the floor of the mouth. They are bound laterally by the mandibular bone and inferolaterally by the mylohyoid muscle. The glands behind each mandibular canine are palpable. Put one index finger into the mouth and the other hand's fingertips outside to physically feel the squeezed gland between the inner and outer fingers. The sublingual gland is composed of approximately twenty minor excretory ducts, also known as ducts of Rivinus, and one major duct [1]. The largest of these, the sublingual duct (of Bartholin), joins the submandibular duct and drains through the sublingual caruncle.
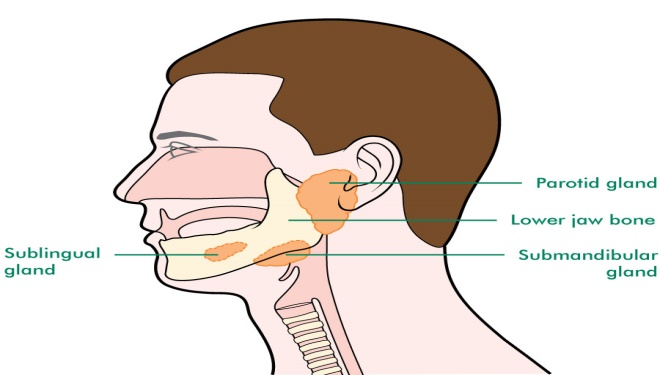
Fig 1: Sublingual Gland
Mechanism of sublingual absortion:The permeability of the solution, also referred to as osmosis, the drug's molecular weight, ionization, and lipid solubility all have an impact on absorption. The medication is absorbed by the oral epithelium's cells through the mechanism of endocytosis. The stratified epithelium is unlikely to exhibit the same mechanism throughout. Nonetheless, it's thought that stimulating the salivary glands acidically and causing vasodilatation in the process helps promote absorption and uptake into the circulatory system [3]. There are mucous glands and squamous epithelium covering the mucous membrane that lines the mouth. The buccal mucosa and sublingual mucosal tissue are comparable.The salivary ducts allow saliva to be secreted into the mouth by lobules of cells that make up the salivary glands [4].
A. Advantages: [2],[5]
- Effects of food are avoided.
- Amplified patient compliance.
- Easy self-medication.
- Quick absorption.
- Higher potency.
- Reduced interaction with other medications and food.
- Ease of administration.
B. Disadvantage: [6]
- Difficulty in keeping the medication underneath your tongue.
- Inability to swallow sublingual medications.
- Inability to administer sublingual medications for all medications.
- Irritation of open sores in the mouth by the medication.
C. Sublingual tablets are special because they:
- Work quickly by dissolving under the tongue.
- Bypass the stomach and liver, leading to faster effects.
- Are easy to take without water.
- Have higher effectiveness at lower doses.
- It is available in various size and shapes.
- It should not be Obstructive.
- It is rapid release.
D. A sublingual tablet should have the following qualities:
- It should dissolve quickly under the tongue.
- The tablet should have a pleasant or neutral taste.
- The drug should be easily absorbed through the mouth tissues.
- The tablet should be small and easy to place under the tongue.
- It should remain stable and effective over time, even in different storage conditions.
- The tablet should not cause irritation or discomfort in the mouth.
- should provide quick relief or effects after dissolving.
E. Factors:
1) Drug Solubility in Lipids:
This is the method by which the drug should be absorbed in its entirety. Its lipid solubility is slightly higher than that of other drugs needed for GI penetration, mostly for passive permeation [7].
2) pH and pKa of saliva:
because saliva has a pH of 7. Hence, pH influences how well a unionized medication is absorbed. In addition, the mucosa's pKa for absorption is greater than 2 in acidic media and less than 8 in basic media.
3) Mucosa epithelial in mouth Thickness:
The sublingual mucosal epithelium's minimum thickness is between 100 and 200 μm. Therefore, because the epithelial mucosa is thinner, medicines permeate more quickly. Therefore, it is simple for a medicine to be absorbed in little saliva.
4) Partition Coefficient:
Sublingual mucosal epithelial cells instantly absorb molecules with an appropriate oil and water partition coefficient. For the best medications, the oil and water partition coefficient can be adjusted within the range of 40 to 2000.
5) Adhesion to the Oral Mucosa:
The medication that binds to the mucosal membrane has low systemic availability.
6) Saliva Solubility:
In addition to having a high lipid solubility, the medication can be dissolved in water in oral fluids. Therefore, the drug's biphasic solubility is essential for absorption.
F. Physicochemical Criteria of drug for Sublingual Drug Delivery:
The essential physico chemical characteristics of a drug for suitable candidate. The formulation of SLTs are summarized in Table [8],[9].
|
Physicochemical Properties of Drug |
Accepted Range |
|
Dose |
< 20 mg |
|
Taste |
Not intensely bitter |
|
Stability |
Good stability in water & saliva |
|
Molecular Weight |
Small to moderate (163.3-342.3g/mol) |
|
pKa |
>2 for acidic Drug; < 10 for basic Drug |
|
Log p |
1.6 to 3.3 |
|
Lipophilicity |
Lipophilic |
Table 1: Physicochemical Criteria of drug for Sublingual Drug Delivery
|
Drug Name |
Brand Name |
Active Ingredient |
Dosage Form |
Manufacturer |
Description |
Market Release |
|
Nitroglycerin |
Nitrostat |
Nitroglycerin |
Sublingual Tablet |
Pfizer |
Used to treat angina; helps relax and widen blood vessels. |
Old (1960s) |
|
Lorazepam |
Ativan |
Lorazepam |
Sublingual Tablet |
Valeant Pharmaceuticals |
Used for anxiety; provides quick absorption through mucous membranes. |
Old (1980s) |
|
Vitamin B12 |
Nature's Bounty Sublingual B12 |
Vitamin B12 |
Sublingual Tablet |
Nature’s Bounty |
Used to treat B12 deficiency; facilitates faster absorption. |
Old (2000s) |
|
Midazolam |
Versed |
Midazolam |
Sublingual Tablet |
Roche |
Used for sedation; provides quick onset of action. |
Old (1990s) |
|
Naloxone |
Narcan |
Naloxone |
Sublingual Tablet |
Adapt Pharma |
Used to reverse opioid overdose; offers quick action to counteract effects. |
Latest (2010s) |
|
CBD (Cannabidiol) |
Charlotte’s Web |
Cannabidiol (CBD) |
Sublingual Tablet |
Charlotte’s Web |
Used for managing anxiety, pain, and sleep disorders; rapidly absorbed. |
Latest (2020s) |
|
Melatonin |
Natrol Sublingual Melatonin |
Melatonin |
Sublingual Tablet |
Natrol |
Used for sleep disorders; fast-acting to help regulate sleep-wake cycles. |
Latest (2010s) |
|
Buprenorphine |
Subutex, Bunavail |
Buprenorphine |
Sublingual Tablet |
Indivior |
Used in addiction treatment; provides controlled release for managing withdrawal symptoms. |
Latest (2020s) |
|
Fentanyl |
Sublimaze, Actiq |
Fentanyl |
Sublingual Tablet/Lozenge |
Teva Pharmaceuticals |
Used for severe pain; offers rapid relief through mucous membrane absorption. |
Latest (1990s) |
|
Estradiol |
Estrace |
Estradiol |
Sublingual Tablet |
Allergan |
Used in hormone replacement therapy; provides systemic estrogen. |
Latest (2000s) |
|
Desmopressi |
Minirin |
Desmopressin |
Sublingual Tablet |
Ferring Pharmaceuticals |
Used to treat diabetes insipidus; helps manage symptoms related to low ADH levels. |
Latest (2010s)
|
|
|
|
|
|
|
|
|
Table 2: summarizing different sublingual tablets [10].
II. METHODS
- Direct compression method: This straightforward and most affordable method is frequently used to prepare sublingual dosage forms. The Direct Compression Method works best with medications that are heat labile. Direct compressible and soluble components, lubricant, superdisintegrant (such as crospovidone, microcrystalline cellulose, etc.), dry binder, sweeteners, and flavors are all used in this process [11],[12].
- Compression molding: The tablets made using this technique will melt and disintegrate quickly (in 4 to 11 seconds). This method's drawback is the tablets' low mechanical strength; to address this issue, binders are added to the formulation blend [13]-[16].
- Freeze-drying: This procedure results in tablets with low mechanical strength and is more expensive and time-consuming than direct compression. Tablets generated from this [17]-[20].
- Hot melt extrusion: By homogeneously and consistently combining the ingredients in a twin screw extruder, it is possible to increase the bioavailability and dissolution rate of medication formulations that are poorly soluble in water. This allows for improved mixing, resulting in a homogenous solid with the medication scattered finely [21]-[26].
- Evaluation Parameters: Along with a few specialized tests, the evaluation parameters of the tablets listed in the pharmacopoeias must be evaluated. Once a tablet is created according to a rule, its quality is often determined by the blend's physicochemical characteristics [27].
A.Pre-compression Studies:
The directly compressible tablet blends were evaluated for pre-compression studies to determine their flow and compressibility.
- Angle of Repose (θ): To find the Angle of Repose, the mixture was poured down the walls of a funnel that was positioned so that the lower tip was about 2 cm above a hard surface.The mixture was poured until the pile surface's upper tip made contact with the funnel's lower tip. The following formula is used to determine the θ. θ = Tan –1 h / r .Where, θ = angle of repose, h = height of heap and r = radius of base of heap circle.
- Bulk density (BD): 2 g of each formulation's mix were put into a 10 mL measuring cylinder after being gently shaken to break up any agglomerates that had formed. The volume is recorded as the bulk volume. The following formula was used to determine the BD. BD = powder weight divided /bulk volume.
- Tapped density (TD): The powder was tapped 100 times to get the tapped volume.The TD was calculated by the following equation. TD = Weight of powder / Tapped volume.
- Carr’s Index (CI): The Carr index referred to as Carr's Compressibility Index, measures a powder's flowability and compressibility.The percentage of CI is calculated by the equation below.CI= (TD-BD) / TD×100.
- Hausner’s Ratio (HR): The Hausner ratio is a number that is correlated to the flowability of a powderIt is calculated by the equation. HR = TD / BD . The Limits for powder flow characteristics were mentioned in Table 3.
|
Flow Character |
Angle of Repose (degrees) |
Carr's Index (%) |
Hausner’s Ratio |
|
Excellent |
25 – 30 |
<10 |
1.00 – 1.11 |
|
Good |
31 – 35 |
11 – 15 |
1.12 – 1.18 |
|
Fair |
36 – 40 |
16 – 20 |
1.19 – 1.25 |
|
Possible |
41 – 45 |
21 – 25 |
1.26 – 1.34 |
|
Poor |
46 – 55 |
26 – 31 |
1.35 – 1.45 |
|
Very Poor |
56 – 65 |
32 – 37 |
1.46 – 1.59 |
|
Very Very Poor |
>66 |
>38 |
>1.60 |
Table 3: Limitations on the properties of powder flow
B. Post-compression Studies:
- Weight variation test: Twenty tablets from each formulation were chosen at random, and their individual weights were carefully measured using an electronic balance.
|
Average weight of the tablet |
Acceptable % deviation |
|
80 mg or less |
10% |
|
More than 80 mg but less than 250 mg |
7.5% |
|
250 mg or more |
5% |
Table 4.Weight variation test acceptable limits according to I.P
- Friability test: Using a friabilator set up to run at 25 RPM for four minutes, 20 tablets from each batch were examined for friability. % Friability = (Initial weight – weight after friability) ) /(Initial weight) x 100.
- Hardness test: A hardness tester is used to determine each formulation's tablets' crushing strength. The Pfizer and Monsanto hardness testers are examples of hardness testers.
- Thickness: The thickness of the tablets in each formulation was measured using vernier callipers.
Conclusion
The results of the study showed that sublingual tablets are a more effective method of medication delivery for elderly and pediatric patients, as well as better for patient compliance. Many medications have been developed using sublingual drug delivery, particularly those that need to take effect quickly. These tablets help those who have trouble swallowing. The intended audience now includes people who prefer easy-to-use pills devoid of water. The medication in the tablets is able to enter the systemic circulation through the sublingual capillaries, which causes an immediate onset of action.
References
[1] Khan, Arshad Bashir, Tarun Kingsley, and Preeta Caroline. \"Sublingual tablets and the benefits of the sublingual route of administration.\" Journal of Pharmaceutical Research 16.3 (2017): 257-267. [2] Thulluru, Ashok, Nawaz Mahammed, C. Madhavi, K. Nandini, S. Sirisha, and D. Spandana. \"Sublingual tablets-an updated review.\" Asian Journal of Pharmaceutical Research 9, no. 2 (2019): 97-103. [3] Lea L. Sublingual Administration. Colon Health. 1996; 13. [4] Boer D et. al. Drug absorption by sublingual and rectal routes. British J Anaesthesia. 1984; 56(1): 69-82. [5] Hooda R, Tripathi M, Kapoor K (2012) A review on oral mucosal drug delivery system. Pharma Innovation 1(1): 13-19. [6] Patel P, Makwana S and et al. Sublingual route for the systemic delivery of Ondansetron. Int J of Drug Development & Research. 2011; 3(4): 36-44. [7] Katz M, Barr M. A study of sublingual absorption I. Several factors influencing the rate of adsorption. J Am Pharm Assoc (Baltim). 1955; 44(7): 419-423. [8] Narang, Neha, and Jyoti Sharma. \"Sublingual mucosa as a route for systemic drug delivery.\" Int J Pharm Pharm Sci 3.Suppl 2 (2011): 18-22. [9] K. C Panda, A. V Reddy, N. Panda, MD Shamim, M. Habibuddin, K.N Jayaveera. Formulation and evaluation of vilazodone sublingual tablets by using lyophilization technique. Research J. Pharm. and Tech. 2018; 11(1): 267-274. [10] Arshad Bashir Khan, Tarun Kingsley, Preeta Caroline. Sublingual Tablets and the Benefits of the Sublingual Route of Administration. Journal of Pharmaceutical Research. 2017; 16(3): 257-67. [11] Dobetti L, inventor; Eurand International SPA, assignee. Fast disintegrating tablets. US patent US 6,596,311. 2003 Jul 22. [12] Van Scoik KG, inventor; Abbott Laboratories, assignee. Solid pharmaceutical dosage in tablet triturate form and method of producing same. US patent US 5,082,667. 1992 Jan 21. [13] Vaishali D. Sangale, Smita S. Aher, Ravindra B. Saudagar. Fast Dissolving Sublingual Film. Asian J. Res. Pharm. Sci. 2016; 6(3): 153-160. [14] Dobetti L. Fast-melting tablets: Developments and technologies. Phar Tech. 2001; 25(9; SUPP): 44-50. [15] Doelker E. Pharmaceutical dosage forms: Tablets: HA Lieberman, L. Lachman and JB Schwartz (Eds.), in three volumes, 2nd Ed., revised and expanded, Marcel Dekker, New York, 1989–1990, 1990, pp.592–616–560. [16] [Chaudry IA, King RE. Migration of potent drugs in wet granulations. J Pharm Sci. 1972; 61(7):1121-5. [17] Priya Patel, NiravJani, NavinSheth, Paresh Patel. Formulation and Optimization of Metoprolol Succinate Sublingual Tablet by Statistical Optimization Technique. Res. J. Pharm. Dosage Form. & Tech. 2015; 7(1); 30-43. [18] Chang RK, Xiaodi G, Burnside BA, Couch RA. Fast-dissolving tablets. Pharm Tech. 2000; 24(6): 52-8. [19] Di Costanzo MF. T-Mask Technologies. In: Proceedings of the 7th International Glatt Symposium, USA 1997 (p. 1-9). [20] Cousin G, Bruna E, Gendrot E, inventors; Laboratoires Prographarm, assignee. Rapidly disintegratable multiparticular tablet. US patent US 5, 464, 632. 1995 Nov 7. [21] Murakami T. Rapidly disintegrating tablets with saccharides. In Proc Int Symp Cont Bioact Mater. 1999; 26: 855-856. [22] Habib W, Khankari R, Hontz J. Fast-dissolve drug delivery systems. Crit Rev Therap Drug Car Sys. 2000; 17(1): 1-12. [23] Dipti G. Phadtare, Amol R. Pawar, R.B. Saudagar, Govind K.Patil. Formulation and Evaluation of Stable Montelukast Sodium Sublingual Tablet by using Lyophilization Technique. Res. J. Pharm. Dosage Form. & Tech. 2017; 9(1): 06-14. [24] Battu SK, Repka MA, Majumdar S, Rao Y M. Formulation and evaluation of rapidly disintegrating fenoverine tablets: effect of superdisintegrants. Drug Dev Ind Pharm. 2007; 33(11):1225-32. [25] El-Arini SK, Clas SD. Evaluation of disintegration testing of different fast dissolving tablets using the texture analyzer. Pharm Dev Tech. 2002; 7(3): 361-71. [26] Dor PJ, Fix JA. In vitro determination of disintegration time of quick-dissolve tablets using a new method. Pharm Dev Tech. 2000; 5(4): 575-7. [27] Banker GS, Anderson NR, Lachman L, Liberman HA. The Theory and Practice of Industrial Pharmacy, 3rd ed. Mumbai, Varghese Publishing House; 1987, p: 293-94.
Copyright
Copyright © 2025 Tejal Pawar , Raj Chitte , Sanjivani Bachhav, Deepali Deore, Prajakta Bachhav . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET66524
Publish Date : 2025-01-14
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

