Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Review: Advances in Lung Cancer Research
Authors: Santosh Bhagwan Gavhane
DOI Link: https://doi.org/10.22214/ijraset.2023.48715
Certificate: View Certificate
Abstract
Lung cancer is one of the most leading causes of cancer death in the world. Lung cancer also known as lung carcinoma is a malignant lung tumor characterized by uncontrolled cell growth in tissues of the lung. If left untreated this growth can spread beyond the lung by process of metastasis into nearby tissue or other parts of the body. Most cancers that start in the lung known as primary lung cancers are carcinomas that derive from epithelial cells. The main primary types are small- cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC). The vast majority (85%) of cases of lung cancer are due to long-term exposure to tobacco smoke. About 10–15% of cases occur in people who have never smoked. These cases are often caused by a combination of genetic factors and exposure to radon gas, asbestos, or other forms of air pollution, including second-hand smoke. This review gives a detailed idea on the epidemiology, causes, types, signs & symptoms, and treatment of lung cancer.
Introduction
I. INTRODUCTION
The top cause of cancer-related death in both men and women worldwide is still lung cancer.
Smoking and using tobacco products are responsible for about 90% of lung cancer cases.
However, additional elements include radon gas, asbestos, exposure to air pollution, and long-term Lung cancer development may be influenced by infections.[1]
There are three main subtypes of NSCLC: adenocarcinoma (30–50% of NSCLC), squamous cell carcinoma, and large-cell carcinoma. NSCLC accounts for about 85% of all lung cancers with a mean survival rate of 15%.
The most prevalent NSCLC (30% of cases) Small cell carcinoma and mixed small cell carcinoma, which combines neoplastic squamous and/or glandular components with small cell carcinoma, are the two subtypes of SCLC. Although NSCLC grows more slowly than SCLC, it spreads more quickly and is more aggressive. In patients with early-stage NSCLC, the recurrence rate is substantial (between 35% and 50%).[2] Five to ten percent of lung tumours are large cell (undifferentiated) carcinomas. This kind of cancer lacks signs of squamous or glandular maturation, hence it is frequently identified by default by ruling out other conditions Possibilities. The core region of the lungs are where large cell carcinoma usually starts, though it can also spread to surrounding lymph nodes, the chest wall, and distant organs. Smoking has a direct correlation with large cell carcinoma tumours.[3] Lung cancer is a late-stage disease for which there is no established screening method, with a median survival of 6–12 months after diagnosis and a 5-year overall survival rate of 5–10%; yet it is known what the main factor is behind this illness. The long-term results of enacting a smoking ban in communities and nations would likely be much greater than what we are currently achieving with all of the available treatments. Even though non-small cell lung cancer patients with surgery have the greatest prognosis, the majority of patients with lung cancer must rely on nonsurgical and adjuvant therapy because the majority of patients are never candidates for curative resection.[4] This review focuses on lung cancer treatments and recent new advances .
II. EPIDEMIOLOGY
According to the most recent global report on the epidemiology of neoplastic disease lung cancer has the highest mortality rate among the 36 cancer types studied and it is the second most commonly diagnosed cancer type in the world . According to projections in 2020 Based on data from 185 countries the estimated number of diagnosed cases was 2,206,771 (11.4% of all cancers) died while 1,796,144 (18.0%) died.[5] Lung cancer is still the leading cause of cancer incidence and mortality in both men and women worldwide, with over 2 million new cases diagnosed each year and 1.8 million deaths.In 2018 they accounted for 18.4% of all cancer deaths.[6] Lung cancers are divided into two types: small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC) (NSCLC). These cancers differ biologically and as a result in their treatment and prognosis. Total national survival rate Patients with lung cancer have been reported from countries all over the world and in the context of histologic subtype-specific survival. The number of lung cancer patients treated in cancer hospitals is also increasing. common population-based data reporting of this survival is uncommon. However, in terms of SCLC status this may not be the case. Three factors make this problematic: Cancer patients receiving treatment those treated in hospitals have a better health status than those treated elsewhere.
General hospitals cancer hospital survival reports are frequently published restricted to surgical patients and overall lung cancer surviving from population-based data primarily reflects the survival of patients who have undergone surgery. NSCLC patients given that NSCLC accounts for more than 80% of cases of lung cancer As a result of these factors overall lung cancer Data on survival may not be applicable to SCLC patients.[7] Lung cancer is most common in developing countries where cigarette smoking is most prevalent with a 20-fold difference in incidence between regions. While prostate cancer is the most common cancer among men in 104 countries lung cancer is the most common in 37 countries including the United States Russia, China, and a large portion of Eastern Europe, the Middle East, and Southeast Asia . In one country North Korea, lung cancer is the most common cancer among women. Micronesia/Polynesia has the highest population density.The global incidence of lung cancer is 52.2/100,000 cases.among men while Hungary has the highest rate Men have a 77.4/100,000 incidence rate.[8] Lung cancer exemplifies the regional differences reflected in the world's geographical distribution. Fig 1 Men have the highest incidence rates in the Middle and Eastern Europe (per 100,000 53.5) and East Asia (50.4 per 100,000). The incidence is lower in Central and West Africa (2.0 and 1.7 per 100,000, respectively). The incidence rate in women is generally low, and the geographical pattern is slightly different, reflecting a variety of historical exposure to tobacco smoke. North America (33.8) and North Europe (23.7) have the highest estimated rates, with Eastern Asia (19.2) having the lowest rates and Western and Central Africa having the highest estimated rates (respectively 1.1 and 0.8). The geographic samples at death rate have a closer resemblance to factors in the case of lung cancer death due to its high death rate (the total ratio of death is 0.87) and lack of relative variability to live in different regions of the world.[9]
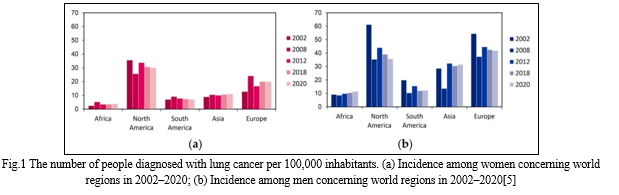
III. TYPE OF LUNG CANCER
Most lung cancers are carcinomas – malignancies arising from epithelial cells. There are two main types of lung carcinoma based on the size and presence of malignant cells as seen by histopathologists under the microscope: non-small cell (80.4%) and small-cell (16.8%) lung carcinoma. This classification, based on histological criteria, has important implications for clinical management and prognosis.[10]
|
Table 1: Types of lung cancer Frequency of histological types of lung cancer
The most frequent malignant lung tumour is, by far, lung cancer. The four primary lung cancer histological subtypes are as follows:
- Squamous cell carcinoma (30% to 40% of lung cancers)
- Adenocarcinoma (25% to 30%)
- Non-small cell lung carcinoma (less than 10%), and
- Small cell lung carcinoma (15% to 20%).
These four types have been divided into several subtypes (1). Bronchoalveolar carcinoma (synonym: alveolar cell carcinoma) sub type of adenocarcinoma that lines the alveoli as it grows. Lung cancer can be classified according to different criteria. Small cell lung carcinoma (15 to 20%) and non-small cell lung carcinoma differ histologically due to their biological differences and the implications of these differences for treatment and diagnosis[11]
IV. SYMPTOMS
In the early stages, lung cancer typically doesn't show any signs or symptoms. Once the condition is advanced, carcinoma signs and symptoms start to appear. The following are some signs and symptoms of carcinoma: [12]
Shortness of breath [13]
Chest discomfort;[12][13]
- Hoarseness
- Bone discomfort
- Headache
- Constant pain
V. RISK FACTOR
The greatest significant risk factor for most malignancies is growing older. The following are other lung cancer risk factors:
- Smoking cigarettes[13]
- Consumption of alcohol[13]
- Exposure to radiation from any of the following sources:[14]
- Breast or chest radiation therapy.
- Workplace or residential radon exposure.
- Diagnostic imaging procedures like computed tomography (CT) scans.
- Radiation from atomic bombs.
- Being surrounded by polluted air.
- Lung cancer in the family history.
- Infection with the human immunodeficiency virus.
- Beta carotene pills for smokers who smoke a lot.
The single most important risk factor for the development of lung cancer is smoking. For smokers the risk of lung cancer is on average tenfold higher than in lifetime non-smokers (defined as a person who has smoked <100 cigarettes in his or her lifetime). The risk increases with the quantity of cigarettes, duration of smoking, and starting age. Smoking cessation results in a decrease in precancerous lesions and a reduction in the risk of developing lung cancer.For years after quitting, former smokers still have an increased chance of developing lung cancer. Smoking with asbestos exposure may increase the risk of lung cancer in a synergistic manner. In addition, there is a 1% to 2% risk per patient per year that a second lung cancer will develop after resection of a lung cancer.[14]
VI. LUNG CANCER TREATMENT
Treatment differs according to the histologic type of cancer, the stage at presentation, and the patient’s functional evaluation
|
Tab 1. Treatment of Lung Cancer According to Stage[15]
A. Surgery
While surgery is the primary treatment for early and mid-stage lung cancer, patients with locally advanced lung cancer may miss out on surgery.disease unless an effective neoadjuvant or adjuvant is available treatment.[16] Patients with stage I, II, and IIIA NSCLC typically undergo surgery to remove the tumour if it is found to be resectable and the patient is able to tolerate it. surgery. Surgeons may remove a lobe or a section of the lung containing the tumour To determine whether or not the tumour is resectable imaging studies and biopsies have been complete as well as an assessment of patient factors to determine operability. Currently, many surgeons use video-assisted surgery.thorascopic surgery (VATS), in which a small incision is made in the chest and a thorascope is inserted.[3] In the last decade lung resection via video-assisted thoracoscopic access has gained widespread acceptance. The new lung adenocarcinoma classification has significant surgical implications as the role of limited resection for early stage lesions is reconsidered. In the early stages of disease, surgery is curative. However, the role of surgery in locally advanced NSCLC is still debatable. The primary goal is a complete resection because this will determine the long-term prognosis. Intra-operative staging of lung cancer is critical for determining the extent of resection based on the tumour and nodal status. Systematic nodal dissection is generally recommended to obtain accurate intra-operative staging and to aid in the decision-making process for adjuvant therapy. Lung resection via video-assisted thoracoscopy has gained widespread acceptance.[17]
B. Chemotherapy
Chemotherapy is the use of chemicals or medications to destroy cancer cells with systemic consequences. So far anticancer medicines are classified into many types depending on their mechanism of action. They include the following methods of action
- Alkylatinging chemicals that cause DNA damage
- Anti-metabolites that replace the regular RNA and DNA building units
- Antibiotics interfere with the enzymes responsible for DNA replication
- Topoisomerase inhibitors that target either topoisomerase I or II are the enzymes responsible for unwinding DNA during replication and transcription;
- Mitotic inhibitors which prevent mitosis and cell division; and
- Corticosteroids, which are used to treat inflammation to treat cancer and to alleviate the negative effects of other medications[18]
At the time of diagnosis, small cell lung cancer is likely to have spread or at least locally advanced, making early resection doubtful. Chemotherapy is the cornerstone of treatment. Various cytotoxic medicines have been the subject of several studies. Combinations and dose regimens have shown that, as long as at least two active medications are taken for at least four weeks, the majority of regimens yield effects that are comparable. Cycles Since the bulk of therapy is now delivered as outpatient care, significant strides have been made in reducing the harmful effects of the treatment. The patient may choose the treatment plan. Cisplatin may not be recommended if you have comorbid conditions such severe peripheral neuropathy or reduced renal function. due to the need for excessive hydration in platinum based.[19]
Both univariate (Figure 1) and multivariate (Table 2) analyses showed that age was inversely associated with the likelihood of receiving chemotherapy (odds ratio).
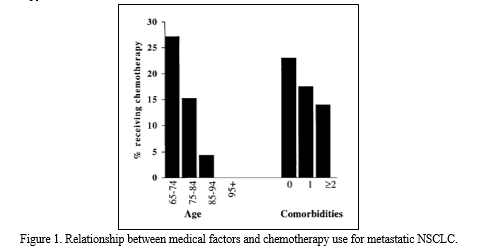
Throughout each decade of life, there is a greater chance of having chemotherapy; African Americans are more likely than non-Hispanic Whites to receive chemotherapy; SES: the proportion of patients who will get chemotherapy as their socioeconomic class rises with each quintile; Seattle/Puget Sound, NM, Seattle/Puget Sound, UT, Los Angeles: When compared to an Atlanta registry participant with intermediate usage, a person residing in the NM/Puget Sound, UT, Los Angeles, or Seattle/Puget Sound SEER registry area was more likely to undergo chemotherapy (after chemotherapy); With each extra comorbidity in the Charlson index, there is a higher chance that a patient may receive chemotherapy; as well as instructing: the potential for chemotherapy if they receive some care at a hospital associated with a medical school[20]
Table 2—Multiple Logistic Regression Analysis
Predicting the Overall Odds of Chemotherapy Use for
Metastatic NSCLC*
|
Variables OR† 95% CI p Value |
|
Intercept – – 0.0001 |
|
Age 0.46 (0.41, 0.51) 0.0001 |
|
Black 0.70 (0.55, 0.88) 0.0032 |
|
SES 1.07 (1.02, 1.12) 0.0077 |
|
UT 0.28 (0.13, 0.50) 0.0001 |
|
NM 0.63 (0.42, 0.93) 0.0234 |
|
Seattle/Puget Sound 1.50 (1.25, 1.80) 0.0001 |
|
Los Angeles 1.65 (1.36, 2.00) 0.0001 |
|
Charlson index 0.85 (0.79, 0.92) 0.0001 |
|
Teaching 1.40 (1.23, 1.60) 0.0001 |
*SES 5 socioeconomic status.
C. Radiotherapy
The impact of advanced RT technology is perhaps most evident in the setting of early-stage NSCLC. Stereotactic ablative radiotherapy (SABR) is now considered the standard of care for medically inoperable patients with peripheral early-stage NSCLC. SABR utilizes small margins for positional uncertainty, facilitated by 4-dimensional computed tomography (4DCT), multiple conformal or intensity modulated beams or arcs and volumetric image-guidance. While peripheral lung SABR can also be delivered without these technologies, newer techniques can increase treatment efficiency and user confidence. Treatment-related toxicity with peripheral lung SABR is modest.
As SABR is not universally available, it is reassuring that data from the randomized SPACE study in patients with peripheral NSCLC suggest similar tumor outcomes with conventionally fractionated 3-dimensional conformal to 70 Gy of radiation.[21] Although surgery is the preferred treatment for early-stage lung cancer in patients who are not candidates for surgery ("medically unfit") or who refuse surgery due to co-existing conditions, experience has shown that radiotherapy has generally been shown to be effective for local attainment.
Control some. Long life data show that medically incompetent patients still die primarily of lung cancer, regardless of their other medical problems, thus supporting supportive care versus curative tumor. Qiaoetal in 1988 and. 2000 reviewed 18 articles. Local recurrence was reported as the predominant 'main mode' of failure and occurred at an average rate of 40%. Median survival in these studies was 18 to 33 months.[22]
D. Immunotherapy
The greater understanding of lung cancer biology has led to the development of many effective targeted therapies as well as of immunotherapy. Immune checkpoint inhibitors (ICIs) have shown tremendous benefit in the treatment of non-small cell lung cancer (NSCLC)[23]
Advanced NSCLC was once considered to be a single disease. NSCLC is now understood to be a biologically complex collection of several illnesses. With multiple authorised targeted medicines, targeted therapy are the norm in NSCLC with a driver mutation. Immunotherapy in the form of immune checkpoint inhibitors (ICIs) is currently a crucial component of the treatment for individuals lacking a driving mutation.[24]
However, following decades of stagnation, the FDA approved the PD-L1 inhibitors atezolizumab and durvalumab in combination with chemotherapy in March of 2019 and 2020, respectively. This permanently altered the treatment landscape. These approvals were based on evidence from two controlled trials, IMpower133 and CASPIAN, which were randomised against chemotherapy’s accepted practise.
Atezolizumab plus etoposide and carboplatin showed increased overall survival (OS) in IMpower133, with a median OS of 12.3 months vs to 10.3 months for etoposide and carboplatin alone (hazard ratio [HR] 0.70; 95% confidence interval [CI], 0.54-0.91; p =.0069).[24]
E. Immune Checkpoints Blockade
Immune checkpoint blockade’ for cancer describes the use of therapeutic antibodies that disrupt negative immune regulatory checkpoints and unleash pre-existing antitumour immune responses.[25]The immune system has developed a range of internal mechanisms, or “checkpoints,” that are employed to limit the intensity and duration of the immune response in order to minimise any unintended harm from activated T cells to surrounding tissues.
T cell activation and inhibition are receptor/ligand processes, which means that agonistic or antagonistic antibodies can control these interactions. Cancer immunotherapy using antibody-mediated immune checkpoint inhibition has advanced significantly during the past ten years.[26]
F. Programmed cell death-1 (PD-1) and PD-L1
For NSCLC therapy, the PD-1 immune checkpoint pathway represents an additional target. It plays a part in reducing T cell activation by lowering immune system activity, which increases self-tolerance and decreases autoimmune disease. In the end, inhibiting this pathway enhances the body’s T cell response. Two drugs, nivolumab and MK-3475, have shown some promising results in early clinical trials.[3]
The Interferon signalling in adaptive programmed cell death 1 ligand 1 expression is shown in fig.1
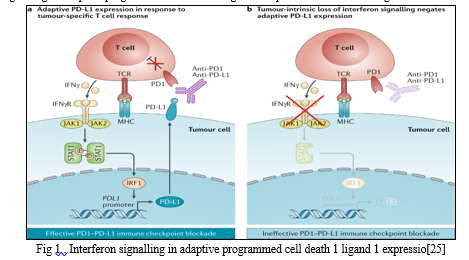
To prevent any unwanted damage caused by activated T cells to surrounding tissues, the immune system has evolved a variety of inbuilt mechanisms, or ‘check-points’that are used to modulate the duration and amplitude of the immune response
To prevent any unwanted damage caused by activated T cells to surrounding tissues, the immune system has evolved a variety of inbuilt mechanisms, or ‘check-points’that are used to modulate the duration and amplitude of the immune responseo prevent any unwanted damage caused by activated T cells to surrounding tissues, the immune system has evolved a variety of inbuilt mechanisms, or ‘check-points’that are used to modulate the duration and amplitude of the immune response.
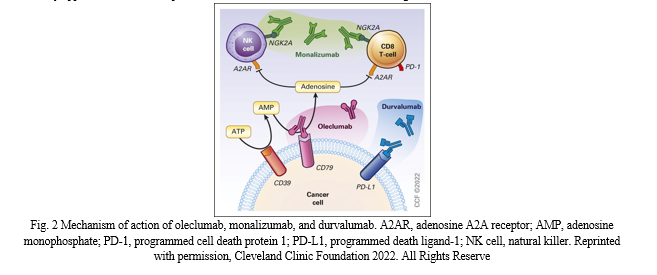
The post Pacific test sample the coast had three arms that hold uncontrolled patients, stage 3 NSCLC which had completed the typical CRT, was randomly assigned either for urvalab consolidation or in combination with one of the two experimental agents, oleculumab or monalizumab. Olecalimumab is a human immunoglobulin G1 targeting CD73, thus reducing the production of immunosuppressant molecules adenosine while the monizumab is one of the nonactivated immunoglobulin G4, a negative regulatory receptor (Figure 2) on immune cells. Both new immunotherapy compounds are being tested together with cell death protein 1/PD-L1 in different settings and programmed agents, a growing general outlook of A recent report by one, now there are more than 5,600 clinical trials of immunotherapy drugs in oncology 7 but only 35% of the cancer drugs reached the stage with 35% are finally approved .It is more important than ever before to determine the initial signs of effectiveness[27]
G. Nivolumab
A completely human igG4 antibody called nivolumab targets PD-1. Nivolumab has an intravenous administration method with a mean half-life of 25 days. In patients with renal failure and mild to moderate hepatic impairment, no dose change is advised. Fatigue, rash, musculoskeletal pain, pruritus, diarrhoea, nausea, asthenia, cough, dyspnea, constipation, decreased appetite, back pain, arthralgia, upper respiratory tract infection, pyrexia, headache, abdominal pain, and vomiting are the most frequent adverse reactions in patients treated with Nivolumab as a single agent. There have been reports of immune-mediated adverse effects including as pneumonitis, thyroiditis, which can cause hypo- or hyperthyroidism, colitis, hepatitis, and nephritis.[28]
H. Pembrolizumab
Pembrolizumab (PD-1 inhibitor) has shown improvement in this disease. The function of PDL-1 is performed in patients regardless of free survival (DFS).The stage was performed for IB-IIIA NSCLC (NCT02504372);Main instructions 091). The risk of recurrent or death in patients diagnosed with random 1177 patients (medium, 53.6 months vs. 42 months. HR = 0.76; 95%CI, 0.63 – 0.91). PD-L1 reverses to a favourable trend in OS by ignoring the expression and some instances have not yet reached a statistical significance. Two years of follow-up with 81.2% of patients receiving Keytruda counting-free compared to 72.8% of patients on placebo. Updated on 2nd August 2019 at Hermione various subgroups were presented with the analysis of epidemiology. The Republican Society of Clinical Oncology (ASCO, 2022) meeting.[29] Food and Drug Administration as single agents in the second-line setting of treatment and then they have been approved in the first-line setting: as single agents in PDL-1 positive patients(pembrolizumab and atezolizumab) in combination with chemotherapy in patients with any histology(pembrolizumab) or non-squamous histology (atezolizumab) or in combination with ipilimumab in PDL-1 positive patients or with ipilimumab and two cycles of chemotherapy (nivolumab). Finally durvalumab has been approved in unresectable stage III NSCLC whose disease has not progressed.[30]
Conclusion
Lung cancer is worldwide major cause of death. As current treatments not advanced t treat stage 3,4 stage patients. Some recent advances in chemotherapy, radiotherapy & newly approved FDA drugs in 2021 brings some hope in Oncology. Still developed nations struggle to treat last stage patients. And poor & middle income/ developing country’s condition is worse. The diagnostic procedures are nit that developed & awareness among people is less. This review put some important aspects about research related to lung cancer. Long run trials, side effects & expenses in developing drugs are some real challenges. Hope the future of oncology will be bright as it is very active area of research in medical field.
References
[1] Lemjabbar-Alaoui, H., Hassan, O.U., Yang, Y.W. and Buchanan, P., 2015. Lung cancer: Biology and treatment options. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer, 1856(2), pp.189-210. [2] Kotiyal, S. and Bhattacharya, S., 2015. Lung Cancer Stem Cells and Their Therapeutic Targeting. [3] Zappa, C. and Mousa, S.A., 2016. Non-small cell lung cancer: current treatment and future advances. Translational lung cancer research, 5(3), p.288. [4] Spiro, S.G. and Porter, J.C., 2002. Lung cancer—where are we today? Current advances in staging and nonsurgical treatment. American journal of respiratory and critical care medicine, 166(9), pp.1166-1196. [5] Rodak, O., Peris-Díaz, M.D., Olbromski, M., Podhorska-Oko?ów, M. and Dzi?giel, P., 2021. Current landscape of non-small cell lung cancer: epidemiology, histological classification, targeted therapies, and immunotherapy. Cancers, 13(18), p.4705. [6] Casal-Mouriño, A., Ruano-Ravina, A., Lorenzo-González, M., Rodríguez-Martínez, Á., Giraldo-Osorio, A., Varela-Lema, L., Pereiro-Brea, T., Barros-Dios, J.M., Valdés-Cuadrado, L. and Pérez-Ríos, M., 2021. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Translational Lung Cancer Research, 10(1), p.506. [7] Oze, I., Ito, H., Nishino, Y., Hattori, M., Nakayama, T., Miyashiro, I., Matsuo, K. and Ito, Y., 2019. Trends in small-cell lung cancer survival in 1993–2006 based on population-based cancer registry data in Japan. Journal of epidemiology, 29(9), pp.347-353 [8] Thandra, K.C., Barsouk, A., Saginala, K., Aluru, J.S. and Barsouk, A., 2021. Epidemiology of lung cancer. Contemporary Oncology/Wspó?czesna Onkologia, 25(1), pp.45-52 [9] Yousheng Mao (2016) Epidemiology of lung cancer Surg Oncol Clin N Am 25 [10] Pandi, A., Mamo, G., Getachew, D., Lemessa, F., Kalappan, V.M. and Dhiravidamani, S., 2016. A brief review on lung cancer. Int. J. Pharma Res. Health Sci, 4, pp.907-914. [11] Hammerschmidt, S. and Wirtz, H., 2009. Lung cancer: current diagnosis and treatment. Deutsches Ärzteblatt International, 106(49), p.809 [12] Xing, P.Y., Zhu, Y.X., Wang, L., Hui, Z.G., Liu, S.M., Ren, J.S., Zhang, Y., Song, Y., Liu, C.C., Huang, Y.C. and Liao, X.Z., 2019. What are the clinical symptoms and physical signs for non?small cell lung cancer before diagnosis is made? A nation?wide multicenter 10?year retrospective study in China. Cancer medicine, 8(8), pp.4055-4069. [13] Tenore, G., Nuvoli, A., Mohsen, A., Cassoni, A., Battisti, A., Terenzi, V., Della Monaca, M., Raponi, I., Brauner, E., De Felice, F. and Musio, D., 2020. Tobacco, alcohol and family history of cancer as risk factors of Oral Squamous Cell Carcinoma: Case-control retrospective study. Applied Sciences, 10(11), p.3896. [14] PDQ Adult Treatment Editorial Board. Non-Small Cell Lung Cancer Treatment (PDQ®): Health Professional Version. 2022 Mar 17. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. [15] Collins, L.G., Haines, C., Perkel, R. and Enck, R.E., 2007. Lung cancer: diagnosis and management. American family physician, 75(1), pp.56-63. [16] Duan, J., Tan, F., Bi, N., Chen, C., Chen, K.N., Cheng, Y., Chu, Q., Ge, D., Hu, J., Huang, Y. and Jiang, T., 2022. Expert consensus on perioperative treatment for non-small cell lung cancer. Translational lung cancer research, 11(7), p.1247. [17] Wang, X. and Han, B., 2013. Advances in lung cancer and treatment research. [18] Huang, C.Y., Ju, D.T., Chang, C.F., Reddy, P.M. and Velmurugan, B.K., 2017. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine, 7(4). [19] Cooper, S. and Spiro, S.G., 2006. Small cell lung cancer: treatment review. Respirology, 11(3), pp.241-248. [20] Earle, C.C., Venditti, L.N., Neumann, P.J., Gelber, R.D., Weinstein, M.C., Potosky, A.L. and Weeks, J.C., 2000. Who gets chemotherapy for metastatic lung cancer?. Chest, 117(5), pp.1239-1246. [21] Baker, S., Dahele, M., Lagerwaard, F.J. and Senan, S., 2016. A critical review of recent developments in radiotherapy for non-small cell lung cancer. Radiation oncology, 11(1), pp.1-14. [22] Scott, W.J., Howington, J., Feigenberg, S., Movsas, B. and Pisters, K., 2007. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines. Chest, 132(3), pp.234S-242S. [23] Mamdani, H., Matosevic, S., Khalid, A.B., Durm, G. and Jalal, S.I., 2022. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Frontiers in Immunology, 13. [24] Oronsky, B., Abrouk, N., Caroen, S., Lybeck, M., Guo, X., Wang, X., Yu, Z. and Reid, T., 2022. A 2022 Update on Extensive Stage Small-Cell Lung Cancer (SCLC). Journal of Cancer, 13(9), p.2945. [25] Kalbasi, A. and Ribas, A., 2020. Tumour-intrinsic resistance to immune checkpoint blockade. Nature Reviews Immunology, 20(1), pp.25-39. [26] Steven, A., Fisher, S.A. and Robinson, B.W., 2016. Immunotherapy for lung cancer. Respirology, 21(5), pp.821-833. [27] Pennell, N.A., 2022. Strategies and End Points in the Development of Novel Immunotherapy Trials for Patients With Unresectable, Locally Advanced Non–Small-Cell Lung Cancer. Journal of Clinical Oncology, pp.JCO-22. [28] Nasser, N.J., Gorenberg, M. and Agbarya, A., 2020. First line immunotherapy for non-small cell lung cancer. Pharmaceuticals, 13(11), p.373. [29] Ramanarayanan, J. and Krishnan, G., 2022. Immunotherapy in Early Stage Non-Small Cell Lung Cancer. Advances in Lung Cancer, 11(3), pp.31-44. [30] Esposito, G., Palumbo, G., Carillio, G., Manzo, A., Montanino, A., Sforza, V., Costanzo, R., Sandomenico, C., La Manna, C., Martucci, N. and La Rocca, A., 2020. Immunotherapy in small cell lung cancer. Cancers, 12(9), p.2522.
Copyright
Copyright © 2023 Santosh Bhagwan Gavhane. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET48715
Publish Date : 2023-01-18
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

