Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
Antibiotics Used in Covid-19 And Mucormycosis (Black Fungus)
Authors: Mr. Chintale Deepak Baliram, Mr. Bagwan L. R., Dr. Hingane L. D.
DOI Link: https://doi.org/10.22214/ijraset.2021.39583
Certificate: View Certificate
Abstract
Background: In late December 2019, Chinese health authorities reported an outbreak of pneumonia of unknown origin in Wuhan, Hubei Province. Summary: A few days later, the genome of a novel coronavirus was released. org/t/novel-2019-coronavirus-genome/319; Wuhan- Hu-1, GenBank accession No. MN908947) and made publicly available to the scientific community. This novel coronavirus was provisionally named 2019-nCoV, now SARS-CoV-2 according to the Coronavirus Study Group of the International Committee on Taxonomy of Viruses. SARS-CoV-2 belongs to the Coronaviridae family, Betacoronavirus genus, subgenus Sarbecovirus. Since its discovery, the virus has spread globally, causing thousands of deaths and having an enormous impact on our health systems and economies. In this review, we summarize the current knowledge about the epidemiology, phylogenesis, homology modeling, and molecular diagnostics of SARS-CoV-2. Phylogenetic analysis is essential to understand viral evolution, whereas homology modeling is important for vaccine strategies and therapies. Highly sensitive and specific diagnostic assays are key to case identification, contact tracing, identification of the animal source, and implementation of control measures.
Introduction
I. INTRODUCTION
In December 2019, an outbreak of pneumonia of unknown origin was reported in Wuhan, Hubei Province, China. Most of these cases were epidemiologically linked to the Huanan Seafood Wholesale Market. Inoculation of bronchoalveolar lavage fluid obtained from patients with pneumonia of unknown origin into human airway epithelial cells and Vero E6 and Huh7 cell lines led to the isolation of a novel coronavirus, SARS-CoV-2, previously named 2019-nCov [1]. Coronaviruses belong to the family Coronaviridae and are positive single-stranded RNA viruses surrounded by an envelope. They are divided into four genera.
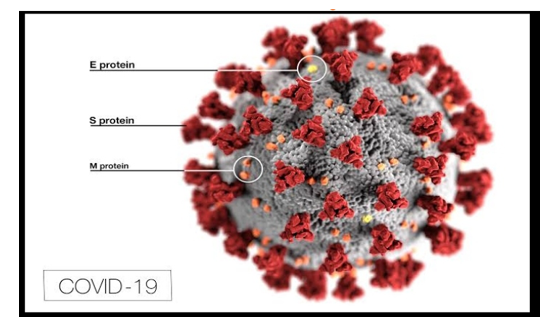
Background Corona virus widely known as COVID-19 are a large family of viruses that are knownto cause illness ranging from the common cold to more severe diseases such as MiddleEast Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS).Figure 1: Sample Picture of Corona VirusImage The virus out-braked on Nov-2019 from Wuhan city of china. The virus affected southChina for about a month and then it spread throughout the world making the paththrough Europe.
The champion’s league game between Atlanta and Valencia becamethe poison for spreading the virus in Europe as more than 80,000 spectators were instadium. Till date, the virus had spread in about 235 territories affecting more than 55million of population.Nepal also reported its first COVID-19 case on 23 January 2020 which today tallied to208,299 cases. The timely imposing of the lockdown controlled the chances of rapidincrementation of infected population till June. But with the foreign manpower beingbrought to home country and loosening of lockdown after July, the number of infectedpopulation increased in the exponential way.The large number of people are being infected and killed on daily basis all over theworld. And the data of death, infected and recovered cases are being provided on
II. ANTIBIOTIC USED IN COVID-19
- Azithromycin
- Doxycycline
- Acyclovir
A. Azithromycin for mild-to-moderate COVID-19
Azithromycin has been widely evaluated for its efficacy against COVID-19. The study by Timothy S C Hinks and colleagues1 adds value to the current literature, and we would like to share our comments.
As stated in the introduction of the Article,1 the antiviral and anti-inflammatory properties of azithromycin are suited to patients with early stage COVID-19. However, patients approaching 14 days since symptom onset might not represent the target study population (ie, those with mild-to-moderate disease), as some patients could be in the late stages of the disease or near resolution by this timepoint. Therefore, it is important to know how many patients were close to 14 days since symptom onset at enrolment, and the rationale behind choosing a 14-day window for inclusion.
It is not clear whether universal pre-determined criteria were used across all participating centres to categorise patients as having mild-to-moderate disease, and whether chest x-rays or CT scans, or both, were done before categorisation. According to the study schedule, participants were enrolled on day 0, received the first dose of azithromycin within 4 h of randomisation, and were followed up on days 14 and 28. To better understand this timeline, it is important to mention if enrolment and randomisation occurred on the same day. We would like to know if the azithromycin regimen (500 mg once daily for 14 days) was based on any pre-established guidelines or recommendations. We consider that it is unreasonable to expose someone with 14 days of symptoms, who might already be near resolution, to a further 14 days of treatment with azithromycin. The study protocol excluded patients with concomitant use of quinolones or macrolides during follow-up; however, additional antibiotics were permitted after randomisation. As concomitant medications can be confounders, their names and indications should be specified.

We had recognised some numerical errors in the older version of the appendix and the Article regarding the total number of participants taking oral corticosteroids. However, we appreciate the authors’ revision, and this error has now been corrected. Due to poor compliance with azithromycin (76 [52%] of 147), a separate subgroup analysis between compliers and non-compliers should be done to further evaluate the effectiveness of this drug. Participants who had received 80% or more doses of azithromycin by day 14 were compliant, but 80% of 28 tablets should be 22, not 24. According to the appendix of the Article,1 76 patients were considered as compliers, but only 73 patients took 28 tablets. We are curious to know how compliance was measured in these three remaining patients. To support the claim that antiviral activity needs to be assessed early in the disease, the authors inappropriately cited their own study protocol.2
Finally, nearly half of the study population included unconfirmed cases of COVID-19, which, in addition to poor compliance to azithromycin, might compromise the validity of the results.
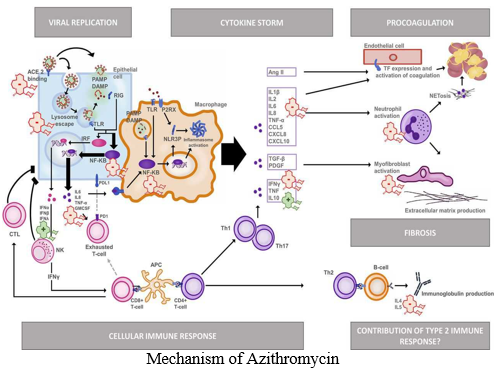
- Mechanism of Azithromycin: It has been demonstrated that in mammalian cells, azithromycin influences intracellular mitogen-activated protein kinase (MAPK), in particular extracellular signal-regulated kinases 1/2 (ERK1/2) and the NF-κB pathway downstream of ERK [12].21-Jul-2020
B. Doxycycline for Community Treatment of Suspected COVID-19
Doxycycline is often used for treating COVID-19 respiratory symptoms in the community despite an absence of evidence from clinical trials to support its use. We aimed to assess the efficacy of doxycycline to treat suspected COVID-19 in the community among people at high risk of adverse outcomes.
We did a national, open-label, multi-arm, adaptive platform randomised trial of interventions against COVID-19 in older people (PRINCIPLE) across primary care centres in the UK. We included people aged 65 years or older, or 50 years or older with comorbidities (weakened immune system, heart disease, hypertension, asthma or lung disease, diabetes, mild hepatic impairment, stroke or neurological problem, and self-reported obesity or body-mass index of 35 kg/m² or greater), who had been unwell (for ≤14 days) with suspected COVID-19 or a positive PCR test for SARS-CoV-2 infection in the community. Participants were randomly assigned using response adaptive randomisation to usual care only, usual care plus oral doxycycline (200 mg on day 1, then 100 mg once daily for the following 6 days), or usual care plus other interventions. The interventions reported in this manuscript are usual care plus doxycycline and usual care only; evaluations of other interventions in this platform trial are ongoing. The coprimary endpoints were time to first self-reported recovery, and hospitalisation or death related to COVID-19, both measured over 28 days from randomisation and analysed by intention to treat. This trial is ongoing and is registered with ISRCTN, 86534580.

In patients with suspected COVID-19 in the community in the UK, who were at high risk of adverse outcomes, treatment with doxycycline was not associated with clinically meaningful reductions in time to recovery or hospital admissions or deaths related to COVID-19, and should not be used as a routine treatment for COVID-19.
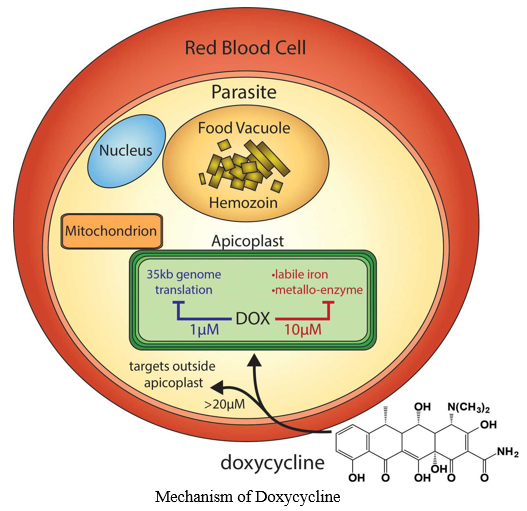
- Mechanism of Doxycycline: Among the tetracycline derivatives, doxycycline is the most potent MMP inhibitor, even at a subantimicrobial dose (25 mg) [34]. As lung immune injury/ARDS is prominent in patients with severe COVID-19, inhibiting MMPs may help repair the damaged lung tissue and enhance recovery [38].06-Jun-2020
C. Acyclovir for SARS-CoV-2: An old drug with a new purpose
Currently, remdesivir is the only Food and Drug Administration approved antiviral for COVID-19. Recent reports of viral mutations in the novel coronavirus are leading to a more infectious agent than at the beginning of the pandemic. Presented in this article are cases that were treated with an old drug, acyclovir. To date, 38 patients have received treatment with acyclovir.
The following 4 cases highlight the benefits of acyclovir. Three of these cases had severe pulmonary disease and one had splenomegaly. One of the three pulmonary cases had worsening pulmonary involvement after hospitalization during which remdesivir in conjunction with dexamethasone was used. Acyclovir has proven to be effective, safe and inexpensive in 29 patients. Nine patients are still under treatment. No adverse effects or death have been observed with this treatment thus far.
Further studies comparing acyclovir to remdesivir are needed to validate benefits from acyclovir for SARS-CoV-2 infection. As of January 1, 2021, the COVID-19 pandemic has resulted in 83,000,000 cases worldwide, with almost 20,000,000 of them in the United States alone, and 1.8 million deaths worldwide, with about 350,000 of them having occurred in the United States [1]. Health officials first noted COVID-19 and its causal agent, SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2), in Wuhan, China. Initial patients had Novel Coronavirus-Infected Pneumonia (NCIP), which persists to this day [2]. COVID-19 patients have also seen severe symptoms like Acute Respiratory Distress Syndrome (ARDS), which causes high levels of mortality [3]. While some patients do not have such complications, the unlucky ones with physician-diagnosed pneumonia, fluidfilled lungs, and ARDS must be hospitalized, and the number of hospitalizations continues
to increase, contributing to an overwhelmed health care system [4]. The National Institutes of Health recommended remdesivir as the only FDA-approved drug for COVID-19 treatment thus far. However, it has also mentioned that treatment plans, including the use of remdesivir, should not be mandated, and the choice to use it rests with the patient and provider [5]. While guidelines promote remdesivir, the drug has come with mixed outcomes and a high cost.
In March 2020, acyclovir was deemed invalid [9]. Since July 2020, our clinic has used acyclovir as a primary antiviral for COVID-19 treatment. Acyclovir is a nucleoside analogue used to treat herpes virus infections, which is selective to the herpes simplex enzyme thymidine kinase.
The drug inhibits viral DNA polymerase through phosphorylation of the acyclovir compound [10]. Acyclovir has been an inexpensive, safe, and well tolerated alternative to treating mild-tosevere cases of COVID-19 for our hospitalized and non-hospitalized patients. The following cases lend support to this statement.
- Mechanism of Acyclovir: Acyclovir is a synthetic analog of the purine nucleoside, guanosine, with potent antiviral activity against herpes simplex viruses type 1 and 2, varicella-zoster virus and other viruses. After conversion in vivo to the active metabolite acyclovir triphosphate, acyclovir competitively inhibits viral DNA polymerase, incorporates into and terminates the growing viral DNA chain, and inactivates viral DNA polymerase. The greater antiviral activity of acyclovir against HSV compared to VZV is due to its more efficient phosphorylation by the thymidine kinase of HSV.
Pacific oyster as a weapon against SARS-CoV-2
Pacific oyster as a
2. Mucormycosis (Black Fungus): Despite the recent introduction of mold-active agents (posaconazole and isavuconazole), in addition to amphotericin B products, to our armamentarium against mucormycosis, many uncertainties remain for the management of this uncommon opportunistic infection, as there are no data from prospective randomized clinical trials to guide therapy. In this mini-review, we present the current status of treatment options. In view of the heterogeneity of the disease (different types of affected hosts, sites of infection, and infecting Mucorales), mucormycosis management requires an individualized management plan that takes into account the net state of immunosuppression of the host, including comorbidities, certainty of diagnosis, site of infection, and antifungal pharmacological properties.
III. MUCORMYCOSIS (BLACK FUNGUS)
A. Antibiotic used Mucormycosis
- Posaconazole: Posaconazole has variable in vitro activity against Mucorales, which is species-dependent. A study of 131 clinical isolates showed that the median MICs of posaconazole for various Mucorales species varied widely between 1.0 and 8.0 _g/mL. In laboratory animal studies, experimental infections produced by Mucor spp. were most responsive to posaconazole, while those caused by Rhizopus spp. were usually non-responsive . Lewis et al. have shown in an immunosuppressed murine model of pulmonary mucormycosis, that a posaconazole serum concentration higher than 4000 _g/mL is needed to suppress the growth of Rhizopus spp. with an MIC of 2 _g/mL . These data raise concerns on the clinical efficacy of posaconazole, at least in the current standard dose of 300 mg/day of extended release tablets, as Rhizopus is among the most common agents causing mucormycosis. Clinical studies on the efficacy of posaconazole for mucormycosis are scarce. Early case reports and case series reported that posaconazole could be an option as salvage therapy in patients unresponsive or intolerant to LAMB. In a subsequent open-label trial including 24 patients, the success rate of salvage therapy with posaconazole oral suspension (800 mg in 4 divided doses) was 70%. The drug was well-tolerated with only minor gastrointestinal side-effects . In another retrospective study of posaconazole oral suspension as salvage therapy in 91 patients with refractory mucormycosis, the response rate was 61%, and in the subgroup of patients with the pulmonary form of mucormycosis 65%. An additional 21% of subjects had stable disease at the end of 12 weeks of treatment . Until recently, posaconazole was available only as oral suspension, administered three or four times daily, with food (preferably a high-fat meal) or with an acidic carbonated beverage, in order to enhance bioavailability. These food requirements make difficult the use of the oral solution in critically ill patients, as they might not be able to eat or they might be nauseous [. Therefore, absorption of posaconazole oral suspension was often suboptimal leading to therapeutic failures. To overcome the pharmacokinetic limitations of the oral solution a gastro-resistant tablet and an intravenous (IV) solution has been developed . The advantages of the tablet formulation over the suspension include better bioavailability allowing once-daily dosage, no food requirements, absorption unaffected by changes in gastric pH or motility; less interpatient variability and more predictable plasma concentrations than the suspension . Despite improved pharmacokinetics, therapeutic drug monitoring (TDM) is suggested for the tablets as it is the case for the suspension formulation . For the rare clinical scenario where patients are unable to take oral drugs, an intravenous formulation of posaconazole containing _-cyclodextrin had been developed, with excellent pharmacokinetic properties. The role of the tablet and IV formulations in the treatment of mucormycosis has not been defined. Currently, posaconazole (oral suspension 400 mg _ 2/day when taken with meals, or 200 mg _ 4/day if not taken with meals) may be considered as salvage treatment of mucormycosis. First–line treatment with posaconazole is considered only in cases when treatment with AMB is absolutely contraindicated , although isavuconazole might be a better option in this situation, as primary treatment data exist only for this newer azole .
- Isavuconazole: Isavuconazole is a new broad-spectrum triazole and is the biologically active agent of the prodrug isavuconazonium sulfate. It is approved in the United States for the treatment of mucormycosis, and in Europe for the treatment of mucormycosis when amphotericin B is not feasible. It is available in both intravenous and oral formulations and it is administered with a loading dose of 200 mg three times a day for two days and 200 mg daily thereafter. Isavuconazole has many pharmacokinetic and safety advantages compared to other azoles, including linear pharmacokinetics and thus no need for TDM; less drug–drug interactions; less toxicity, especially hepatotoxicity, skin and ocular side-effects, or QT prolongation; no nephrotoxic cyclodextrin in the IV formulation; no need for dose adjustment in kidney, liver failure or obesity; and excellent oral bioavailability with no food requirements As it is the case with posaconazole, isavuconazole has variable, species-dependent, in vitro activity against Mucorales [56,59]. It should be noted that the MIC values of isavuconazole for Mucorales are 2- to 4-fold higher compared to those of posaconazole, and this should be taken into account in clinical practice. In the neutropenic mouse model of mucormycosis due to Rhizopus, isavuconazole had comparable efficacy to high-dose L-AmB in reduction of tissue fungal burden, in both the lung and the brain, and provided a survival benefit at 21 days of treatment. Several case reports have shown that isavuconazole could be used successfully as salvage therapy for mucormycosis, in heavily immunosuppressed patients, including cases of posaconazole failure.
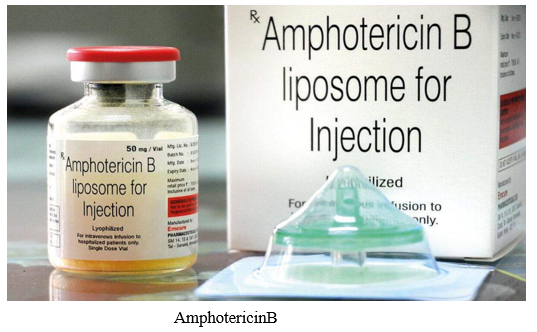
- Amphotericin B: AMB is considered the drug of choice for primary treatment of mucormycosis. The efficacy of AMB has been shown in both laboratory (in vitro and in vivo) and clinical studies. Although interpretive breakpoints to AMB have not been determined, high in vitro MICs to AMB have been observed in clinical isolates of Cunninghamella species. However, in a small study of non-Aspergillus invasive mould infections, an MIC for amphotericin B of _0.5 _g/mL was significantly associated with better 6-week outcomes. Lipid formulations of amphotericin B (liposomal AMB, LAMB; and AMB lipid complex, ABLC) have better therapeutic index than the conventional amphotericin B deoxycholate and are considered as the first-line therapy of mucormycosis. As with many antifungal agents and mycoses, the optimal dosage for AMB and its formulations against mucormycosis is still undetermined. The standard daily dose of LAMB and ABLC suggested by current guidelines is 5 mg/kg/day . The in vitro activity of AMB against Mucorales is highly variable. Recently, researchers reported that among 524 clinical Mucorales isolates, the epidemiologic cut-off values (ECVs) _97.5% for amphotericin B were rather high: 2 _g/mL for L. corymbifera, 2 _g/mL for M. circinelloides, 4 _g/mL for R. arrhizus, and 2 _g/mL for R. microspores . These relatively high AMB MIC values support the use of higher daily dose of AMB to achieve clearance of Mucorales from tissues. Indeed, in a neutropenic murine model of pulmonary mucormycosis, the efficacy of liposomal AMB was dose-dependent: a dose of 10 mg/kg/day has been proved to be more effective in reducing fungal burden compared to 5 or 1 mg/kg/day. Based on these in vitro and in vivo data, researchers proposed to treat mucormycosis with high dose LAMB (>5 mg/kg/day). Yet, this recommendation has not been supported by the findings of asubsequent clinical study. The French Mycoses Study Group conducted a phase I–II prospective, multicenter, pilot trial on the efficacy and safety of high-dose (10 mg/kg/day) LAMB monotherapy (AmBizygo study) for the treatment of mucormycosis. The study included 40 patients, the majority of them with underlying hematological malignancy and/or HSCT. Surgery and debridement has been performed in 71% of the patients before initiation of antifungal therapy. Compared to historical controls receiving the standard dose of 5 mg/kg/day, no improvements in mortality and response rates (38 and 36% respectively) was seen at 12 weeks of treatment. On the other hand, high dose L-AMB was associated with increased nephrotoxicity and electrolyte derangements. Characteristically, doubling of the baseline serum creatinine levels has been observed in 40% of the patients, dictating dose reduction. Although dosages beyond 5 mg/kg/day have not been proved to be more efficacious for mucormycosis, they may be considered on an individual basis, especially when there is CNS or osteoarticular involvement .
References
[1] Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al.; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb; 382(8): 727–33. [2] Almeida JD, Tyrrell DA. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture. J Gen Virol. 1967 Apr; 1(2): 175–8. [3] Kapikian AZ, James HD Jr, Kelly SJ, Dees JH, Turner HC, McIntosh K, et al. Isolation from man of “avian infectious bronchitis viruslike” viruses (coronaviruses) similar to 229E virus, with some epidemiological observations. J Infect Dis. 1969 Mar; 119(3): 282–90. [4] Peiris JS, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004 Dec; 10(12 Suppl):S88–97. [5] van der Hoek L, Pyrc K, Jebbink MF, Vermeulen- Oost W, Berkhout RJ, Wolthers KC, et al. Identification of a new human coronavirus. Nat Med. 2004 Apr; 10(4): 368–73. [6] Woo PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005 Jan; 79(2): 884–95. [7] Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012 Nov; 367(19): 1814–20. [8] Wevers BA, van der Hoek L. Recently discovered human coronaviruses. Clin Lab Med. 2009 Dec; 29(4): 715–24. [9] Kwon-Chung, K.J. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: Molecular mycologic perspectives. Clin. Infect. Dis. 2012, 54 (Suppl. 1), S8–S15. [10] Roden, M.M.; Zaoutis, T.E.; Buchanan,W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. 2005, 41, 634–653. [11] Gomes, M.Z.; Lewis, R.E.; Kontoyiannis, D.P. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin. Microbiol. Rev. 2011, 24, 411–445. [12] Ribes, J.A.; Vanover-Sams, C.L.; Baker, D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2000, 13, 236–301. [13] Andresen, D.; Donaldson, A.; Choo, L.; Knox, A.; Klaassen, M.; Ursic, C.; Vonthethoff, L.; Krilis, S.; Konecny, P. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet 2005, 365, 876–878. [14] Legrand, M.; Gits-Muselli, M.; Boutin, L.; Garcia-Hermoso, D.; Maurel, V.; Soussi, S.; Benyamina, MFerry, A.; Chaussard, M.; Hamane, S.; et al. Detection of circulating Mucorales DNA in critically ill burn patients: Preliminary report of a screening strategy for early diagnosis and treatment. Clin. Infect. Dis. 2016, 63, 1312–1317.
Copyright
Copyright © 2022 Mr. Chintale Deepak Baliram, Mr. Bagwan L. R., Dr. Hingane L. D.. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET39583
Publish Date : 2021-12-22
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

