Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Analysis Report on Artificial Oxygenated System: A Report
Authors: Parmeshwar Gorain, Nakul Dhall, Abhishek Maheshwari, Anjali Gupta, Dr. Deepshika
DOI Link: https://doi.org/10.22214/ijraset.2022.47983
Certificate: View Certificate
Abstract
As we know that nowadays, air pollution is increasing because of gases present in the atmosphere. Gases such as Carbon Dioxide, Methane, Carbon Monoxide are increasing rapidly. So, Air Quality Index is not upto the health mark. So, it is definite that we would be having more breathing problem in the future. There would be the time when we won’t be able to get any oxygen on this planet. So, Our main motive is to reduces such harmful gases and purify the oxygen artificially.
Introduction
I. INTRODUCTION
Pollution, release into the atmosphere of various gases, finely divided solids, or finely dispersed liquid aerosols at rates that exceed the natural capacity of the environment to dissipate and dilute or absorb them. These substances may reach concentrations in the air that cause undesirable health, economic, or aesthetic effects.
Clean, dry air consists primarily of nitrogen and oxygen—78 percent and 21 percent respectively, by volume. The remaining 1 percent is a mixture of other gases, mostly argon (0.9 percent), along with trace (very small) amounts of carbon dioxide, methane, hydrogen, helium, and more. Water vapour is also a normal, though quite variable, component of the atmosphere, normally ranging from 0.01 to 4 percent by volume; under very humid conditions the moisture content of air may be as high as 5 percent.
There are six major air pollutants that have been designated by the U.S. Environmental Protection Agency (EPA) as “criteria” pollutants—criteria meaning that the concentrations of these pollutants af in the atmosphere are useful as indicators of overall air quality.
The gaseous criteria air pollutants of primary concern in urban settings include sulfur dioxide, nitrogen dioxide, and carbon monoxide; these are emitted directly into the air from fossil fuels such as fuel oil, gasoline, and anatural gas that are burned in power plants, automobiles, and other combustion sources. Ozone (a key component of smog) is also a gaseous pollutant; it forms in the atmosphere via complex chemical reactions occurring between nitrogen dioxide aand various volatile organic compounds (e.g., gasoline vapours).
Airborne suspensions of extremely small solid or liquid particles called “particulates” (e.g., soot, dust, smokes, fumes, mists), especially those less than 10 micrometres (μm; millionths of a metre) in size, are significant air pollutants because of their very harmful effects on human health. Except for lead, criteria pollutants are emitted in industrialized countries at very high rates, typically measured in millions of tons per year. All except ozone are discharged directly into the atmosphere from a wide variety of sources. They are regulated primarily by establishing ambient air quality standards, which are af maximum acceptable concentrations of each criteria pollutant in the atmosphere, regardless of its origin. Although pure water is rarely found in nature (because of the strong tendency of water to dissolve other substances), the characterization of water quality (i.e., clean or polluted) is a function of the intended use of the water. For example, water that is clean enough for swimming and fishing may not be clean enough for drinking and cooking. Water aquality standards (limits on the amount of impurities allowed in water intended for a particular use) provide a legal framework for the prevention of water pollution of all types.
II. LITERATURE SURVEY: ARTIFICIAL OXYGENATED SYSTEM
Thermal gases, or GHGs, are composite gases or longwave rays in the atmosphere. The main GHGs, also called thermal gases, are CO2, methane, nitrous oxide, hence af gaseous gases.
Pollution is becoming a major environmental issue in terms of energy efficiency and carbon emissions. the following are the key factors that have enabled Artificial Oxygenated Ecosystem. in this way, organizations can reduce carbon emissions by at least 30-40%.
Cheuk-Yiu Ng gave a new challenge in the field of science, as the oxygen will not going to be forever here so there is a theory of making artificial oxygen to make it by gases or some other chemical substances.
Edward Schwieterman (astrologist, UC) originally proposed a similar way of detecting high concentrations of oxygen from nonliving processes and was a member of the team that developed this technique.
"Oxygen is one of the most exciting molecules to detect because of its link with life, but we don't know if life is the only cause of aoxygen in an atmosphere," Schwieterman said. "This technique will allow us to find oxygen in planets aboth living and dead.
Even though scientists think plants produced most of the oxygen present on Earth, they suspected some oxygen may have existed before photosynthetic organisms arose, said Cheuk-Yiu Ng, a physical chemist at the University of California, Davis, and co-author of the study published today (Oct. 2) in the journal Science.
But, it was thought that the planet's oxygen (O2) formed from two oxygen atoms colliding and combining on some surface, not because the oxygen molecules split from carbon dioxide (CO2), Ng said.
"This machine is unique in the world," Ng said.
When the researchers shone the first laser on the carbon dioxide, the second laser detected O2 molecules and carbon atoms, suggesting aa small amount of carbon dioxide (about 5 percent) was turned into oxygen. Though small, that's enough to show athat it's possible to produce oxygen from CO2 by a nonbiological process, Ng said.
Finally, the researchers hinted that it may be possible to use this technique to make oxygen in space or on other planets. Bu t first , more studies are needed to verify the fundamentals of how this reaction aoccurs, the scientists said. One reason the experiment ahadn't been done before is because of the difficulty of acreating intense vacuum ultraviolet light, Ng said. One way is to use a particle accelerator called a synchrotron, but the laser in Ng's lab is 10,000 to 1 million times abrighter than those produced by existing synchrotrons, he said.
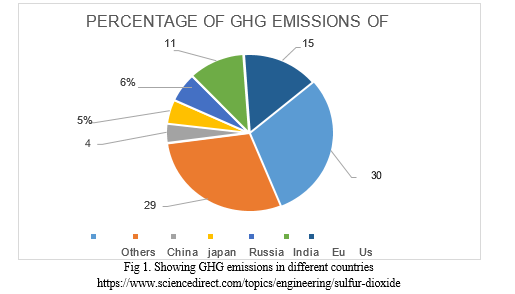
Here is a chart representing carbon emissions in several countries:
A. Why Artificial Oxygen?
As we know that nowadays, air pollution is increasing because of gases present in the atmosphere. Gases such as Carbon Dioxide, Methane, Carbon Monoxide are increasing rapidly. So, Air Quality Index is not upto the health mark. So, it is definite that we would be having more breathing problem in the future. There would be the time when we won’t be able to get any oxygen on this planet. So, for the basic survival, we need to make sure that we have enough oxygen on our planet so that we could just make our artificial environment. In near future, it can be clearly visible that we will be needed the artificial oxygen real quick as most of the oxygen get polluted already.
B. Major Air Pollutants
Very small fragments of solid materials or liquid droplets suspended in air are called particulates. Except for airborne lead, which is treated as a separate category, they are characterized on the basis of size and phase (i.e., solid or liquid) rather than by chemical composition. For example, solid particulates between roughly 1 and 100 μm in diameter are called dust particles, whereas airborne solids less than 1 μm in diameter are called fumes.
Here are some pollutants
- Carbon Monoxide
- Nitrogen Oxide or Oxides
- Sulphur Dioxide
- Ozone
The particulates of most concern with regard to their effects on human health are solids less than 10 μm in diameter, because they can be inhaled deep into the lungs and become trapped in the lower respiratory system. Certain particulates, such as asbestos fibres, are known carcinogens (cancer causing agents), and many carbonaceous particulate s—e.g., soot—are suspected of being carcinogenic. Major sources of particulate emissions include fossil-fuel power plants, manufacturing processes, fossil-fuel residential heating systems, and gasoline-powered vehicles.
Carbon monoxide is an odorless, invisible gas formed as a result of incomplete combustion. It is the most abundant of the criteria pollutants. Gasoline-powered highway vehicles are the primary source, although residential heating systems and certain industrial processes also emit significant amounts of this gas. Power plants emit relatively little carbon monoxide because they are carefully designed and operated to maximize combustion efficiency. Exposure to carbon monoxide can be acutely harmful since it readily displaces oxygen in the bloodstream, leading to asphyxiation at high enough concentrations and exposure times.
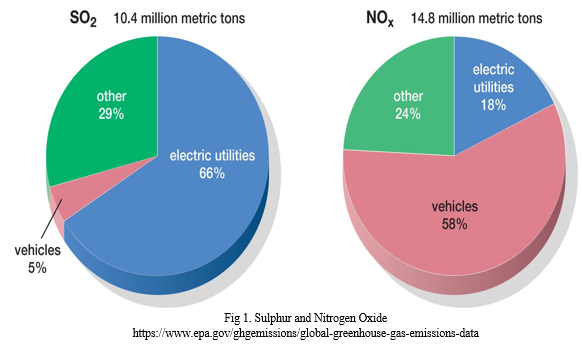
C. Sulphur and Nitrogen Oxide Emission
III. SOURCES AND RISKS OF POLLUTANTS
|
Criteria air pollutants |
||||
|
pollutant |
common sources |
maximum acceptable concentration in the atmosphere |
environmental risks |
human health risks |
|
carbon monoxide (CO) |
automobile emissions, fires, industrial processes |
35 ppm (1-hour period); 9 ppm (8-hour period) |
contributes to smog formation |
exacerbates symptoms of heart disease, such as chest pain; may cause vision problems and reduce physical and mental capabilities in healthy people |
|
nitrogen oxides (NO and NO2) |
automobile emissions, electricity generation, industrial processes |
0.053 ppm (1-year period) |
damage to foliage; contributes to smog formation |
inflammation and irritation of breathing passages |
|
sulfur dioxide (SO2) |
electricity generation, fossil-fuel combustion, industrial processes, automobile emissions |
0.03 ppm (1-year period); 0.14 ppm (24-hour period) |
major cause of haze; contributes to acid rain formation, which subsequently damages foliage, buildings, and monuments; reacts to form particulate matter |
breathing difficulties, particularly for people with asthma and heart disease |
|
ozone (O3) |
nitrogen oxides (NOx) and volatile organic compounds (VOCs) from industrial and automobile emissions, gasoline vapours, chemical solvents, and electrical utilities |
0.075 ppm (8-hour period) |
interferes with the ability of certain plants to respire, leading to increased susceptibility to other environmental stressors (e.g., disease, harsh weather) |
reduced lung function; irritation and inflammation of breathing passages |
|
particulate matter |
sources of primary particles include fires, smokestacks, construction sites, and unpaved roads; sources of secondary particles include reactions between gaseous chemicals emitted by power plants and automobiles |
150 μg/m3 (24-hour period for particles <10 μm); 35 μg/m3 (24-hour period for particles <2.5 μm) |
contributes to formation of haze as well as acid rain, which changes the pH balance of waterways and damages foliage, buildings, and monuments |
irritation of breathing passages, aggravation of asthma, irregular heartbeat |
|
|
|
|
|
|
IV. INNOVATION
A. Artificial Oxygen Techniques
Manufacturing Process
Most commercial oxygen is produced using a variation of the cryogenic distillation process originally developed in 1895. This process produces oxygen that is 99+% pure.
More recently, the more energy-efficient vacuum swing adsorption process has been used for a limited number of applications that do not require oxygen with more than 90-93% purity.
B. The Future Of Data Centers
- Despite the fact that allogeneic red blood cell (RBC) transfusions can be life-saving in exsanguinating trauma patients, many adverse events impacting patient outcome have documented [1]. Therefore, artificial oxygen carriers were initially been developed as “blood substitutes” in the 1980s and 1990s. Artificial oxygen carriers can be grouped into hemoglobin-based oxygen carriers (HBOCs) and perfluorocarbon-based oxygen carriers (PFCs) [2].
- The clinical use of artificial oxygen carriers has mainly been studied in trauma and major surgery [3]. HBOC studies in general did not show a benefit in the primary outcome parameter such as avoidance/reduction of RBC transfusions or 28-day mortality [3] and signs of vasoconstriction/hypertension due to nitric oxide scavenging and increased relative risk of myocardial infarction and death were shown in a meta-analysis [4]. Consequently, the Food and Drug Agency in 2008 put all HBOC trials on hold.
- PFC studies in non-cardiac surgery were successful in reversing physiologic transfusion triggers and in reducing the need for allogeneic RBC transfusions [5]. In addition, there were no major safety issues. However, a PFC study in cardiac surgery was prematurely stopped due to an increased incidence of neurologic adverse events, and this program has never been re-started.
- In recent years, focus on the potential clinical use of artificial oxygen carriers moved away from “blood substitutes” towards “oxygen therapeutics”. Due to the relatively short half-life of 12–24 h, this may indeed be reasonable. However, clinical studies showing clear benefits in this new area are still scarce. The area with most documented evidence are “compassionate use” programs. In such programs, patients were treated with HBOCs at a median hemoglobin concentration of 39 g/l [6]. Survival of patients with severe anemia for whom RBC transfusion was not an option was clearly and significantly higher if treated with an HBOC [7]. It is also conceivable that an HBOC may be capable of bridging a patient with severe anemia until RBC transfusions become available.
- In animal models, artificial oxygen carriers have also proven to be efficacious in relieving organ ischemia such as fetal hypoxia in pre-eclampsia [8] and cerebral ischemia [9]. However, in a recent study myocardial perfusion with an oxygenated HBOC-enriched solution did not reduce the infarct volume nor was post-ischemic cardiac function improved [10]. In contrast, HBOC attenuated intense exercise-induced cardiac dysfunction [11].
- Machine perfusion of liver grafts after prolonged cold ischemia with HBOC enriched perfusate appears to be efficacious in improving the condition of the liver graft prior to transplantation in multiple animal experiments [12]. And recently the first human liver transplantation after machine perfusion with HBOC was performed.Footnote1 The use of artificial oxygen carriers in pre-transplant perfusion is also conceivable in other organs such as lung and heart. The future will tell whether HBOCs or PFCs are more efficacious.
- PFCs may also be used as contrast agents [13] and, in conjunction with magnetic resonance imaging, as infection tracers [14].
- Finally yet importantly, artificial oxygen carriers look like a logical adjunct to Patient Blood Management. Patient Blood Management is already highly successful: a reduction in the use of allogeneic blood product transfusion of approximately 40%, a decrease in hospital mortality (−28%), infection rate (−21%), combined myocardial infarction and stroke (−31%), length of hospital stay (−15%), and annual costs ($7–29 million) has been described in a study on 605,000 patients in Western Australia [15]. Nevertheless, having an artificial oxygen carrier to bridge the period of low hemoglobin/hematocrit or in the context of augmented hemodilution [5] might broaden the spectrum of Patient Blood Management and may make it even more successful.
w.r.f “https://clinicaltrials.gov/”
Conclusion
Global warming is recognized by almost all atmospheric scientists as a significant environmental problem caused by an increase in levels of certain trace gases in the atmosphere since the beginning of the Industrial Revolution in the mid-18th century. These gases, collectively called greenhouse gases, include carbon dioxide, organic chemicals called chlorofluorocarbons (CFCs), methane, nitrous oxide, ozone, and many others. Carbon dioxide, although not the most potent of the greenhouse gases, is the most important because of the huge volumes emitted into the air by combustion of fossil fuels (e.g., gasoline, oil, coal). Carbon dioxide is considered a normal component of the atmosphere, and before the Industrial Revolution the average levels of this gas were about 280 parts per million (ppm). By 2020 the levels of carbon dioxide had reached 417 ppm, and they continue to increase at a rate of almost 3 ppm per year. Many scientists think that carbon dioxide should be regulated as a pollutant—a position taken by the EPA in 2009 in a ruling that such regulations could be promulgated. International cooperation and agreements, such as the Paris Agreement of 2015, would be necessary to reduce carbon dioxide emissions worldwide.
References
[1] https://www.britannica.com/science/air-pollution/Greenhouse-gases [2] https://www.healthcentral.com/article/the-three-types-of-oxygen-therapy-for-copd [3] https://www.livescience.com/48125-oxygen-made-from-carbon-dioxide.html [4] https://jpet.aspetjournals.org/content/jpet/369/2/300.full.pdf [5] https://www.sciencedirect.com/topics/engineering/sulfur-dioxide [6] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC137239/ [7] https://www.scielo.br/j/spmj/a/Zsc3xxk4qb4SPSjv3twb5Gb/?lang=en [8] https://www.ucsfhealth.org/education/your-oxygen-equipment [9] https://ccforum.biomedcentral.com/articles/10.1186/s13054-018-1949-5 [10] https://pubmed.ncbi.nlm.nih.gov/17591302/
Copyright
Copyright © 2022 Parmeshwar Gorain, Nakul Dhall, Abhishek Maheshwari, Anjali Gupta, Dr. Deepshika. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
.jpg)
Download Paper
Paper Id : IJRASET47983
Publish Date : 2022-12-08
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online