Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Isolation, Screening, Characterization and Maintenance of Amylase Producing Bacteria from Soil
Authors: Avinash Gadhave, Mahesh S Wagh, Pravin Kale, Sagar Bhakare
DOI Link: https://doi.org/10.22214/ijraset.2022.46121
Certificate: View Certificate
Abstract
Amylases are amongst most widely used enzymes in industries such as food, fermentation, starch processing, textile, and paper. In the present investigation, bacteria were isolated from soil samples of different areas of Padmashri Vikhe Patil College, Pravaranagar, Maharashtra, India screened for the production of amylase. Soil samples were serially diluted in normal saline and plated on sterile nutrient agar plates. The colonies obtained from higher dilutions were subjected to Gram staining, biochemical reactions, and starch hydrolysis test. Bacterial colony yielding positive starch hydrolysis test were subjected to Amylase activity test by DNSA method. Characteristic feature of the strain indicates that it belongs to the genus Bacillus and will be later used for further characterization. Maximum yield of amylase was obtained after 48hrs of incubation. The isolated amylase producing organism was maintain by using agar slant & glycerol stock method.
Introduction
I. INTRODUCTION
Enzymes are chemical compounds made by living cells that have the ability to start a chemical process without being consumed themselves. They increase a chemical reaction's speed (Oyeleke et al., 2009). Animals and plants both create enzymes, however the creation of microbial enzymes is particularly significant since these more stable, calculable, and cost-effective to make (Burhan et al., 2003).
Glucoamylase, α-amylase, and other starch hydrolytic amylases are some of the most frequently employed enzymes in modern biotechnology. Glucoamylases, also known as amylo-glucosidases, are exo-acting amylases that convert the non-reducing end of starch and associated oligosaccharides into glucose. Commercially available glucose amylases are used to convert malto-oligosaccharides into glucose. Despite the fact that amylases can be produced from a variety of sources (including microbes, plants, and animals), amylases from microbes are best suited for industrial production because of their quick growth cycles, low production costs, environmentally friendly behavior, productivity, and ease of bacterial gene manipulation. Amylases are typically secreted outside of cells by bacteria and fungi to undertake extracellular starch breakdown into sugars.
Due to their specificity of reaction, mild conditions required for the reaction, and lower energy consumption than traditional non-enzymatic chemical techniques, microbial amylases are becoming more and more in demand in industry.
II. MATERIALS & METHODS
A. Sample Collection
Soil samples were collected from different areas of Padmashri Vikhe Patil College, Pravaranagar, Maharashtra, India, with the help of sterile spatula. Collected samples were transferred to sterile plastic bags in aseptic conditions.
B. Isolation of Amylase Producing Microorganism
One gram of the soil sample was weighed and mixed to 9 ml of sterile distilled water. Serial dilution was done up to 10-6 and spread plated into nutrient agar with 1% starch. Then the plates were kept in bacterial incubator at 37°C for 48 hours.
C, Screening for Amylase Activity (Starch Iodine Test)
Bacterial cultures were screened for amylolytic activity by starch hydrolysis test on starch agar plate. The pure isolated colonies were streaked on starch agar plates with starch as the only carbon source.
After incubation at 37ºC for 24-48 hours, the individual plates were flooded with Gram’s iodine solution with the help of dropper to produce a deep blue colored starch-iodine complex. When iodine comes in contact with a medium containing starch, it turns blue. If starch is hydrolyzed, the medium will have a clear zone next growth. A clear zone around the bacterial colony indicates amylase production. The pure cultures were sub cultured at regular intervals by using nutrient agar slants & glycerol stock method were maintained at 4°C.
D. Maintenance of the Isolated Microorganism
- Agar Slant Method: An isolated culture was streaked on nutrient agar plate to obtain well isolated colony. A well isolated colony was selected for sock culture preparation. The nutrient agar medium for the slants were prepared. after distributing in test tubes, the medium was sterilized in autoclave at 121°C for 15 minutes. After sterilization, all the test tubes were placed in a slanting position and allowed it to solidify without any disturbance. Then, suspension of a selected colony was streaked on the sterile nutrient agar slants in zigzag manner form bottom to top. All slants were kept for incubation at 37°C for 24 hours. After incubation, optimum growth of microorganism was observed and all slants were sealed by using parafilm. All slants were labeled properly as master or working slant, name of culture, medium used and date etc. All slants were stored at low temperature in refrigerator in lower compartment.
- Glycerol Stock Method: An isolated culture was allowed to grow overnight in sterile LB broth till its log phase of growth. Sterile glycerol solution (60%) was prepared by diluting glycerol with distilled water and then sterilized at 121°C for 24 hrs. All sterile vials and tip box were kept in freezer for cooling. After incubation the culture was mixed with sterile glycerol into each sterile vial by using micropipette in the proportion aseptically i.e., 900µl of broth culture and 100µl of glycerol stock for 1000µl volume per vial. After addition the content in each vial was mixed by vortexing. All vials were transferred immediately into a deep freezer. For Eppendorf tubes that were used instead of screw cap vials were sealed properly and then transferred into deep freezer.
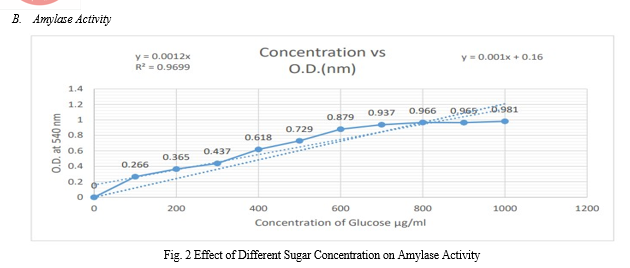
E. Enzyme Assay
The enzyme activity of amylase enzyme can be determined by using DNS method. 1.5 ml of 1% starch in 2 ml, 0.1M phosphate buffer (pH 6.5) and 0.5ml of diluted enzyme were incubated for 15 minutes at room temperature.
The reaction was arrested by adding 1ml of DNS reagent and kept in a boiling water bath for 10 minutes and diluted with 8ml distilled water. The absorbance was measured at 540nm against blank prepared as above without incubation. One unit of α-amylase activity was defined as the amount of enzyme that liberates 1μmole of reducing sugar (maltose equivalents) per minute under the assay conditions.
F. Morphological & Biochemical Characterization
The isolates were observed under the microscope to obtain the colony morphology i.e., color, size, shape, opacity, margin.
- Gram Staining: To test whether the isolated bacteria Gram positive or Gram negative, the Gram staining was performed. A thin smear of pure 24 hrs. old culture was prepared on clean grease-free slide, fixed by passing over gentle flame. Heat-fixed smear was stained by addition of 2 drops of crystal violet solution for 60 sec and rinsed with water. The smear was again flooded with Gram’s iodine for 30 sec and rinsed with water, decolorized with 70% alcohol for 15 sec and rinsed with distilled water. Then counter stained 2 drops of Safranin was applied for 60 sec and finally rinsed with water, then allowed to air dry. The smears were mounted on a microscope and observed under microscope. Gram negative cells appeared pink or red while gram positive organisms appeared purple.
- Motility Test: The Motility test was performed to test whether the isolated bacteria motile or non-motile in nature. A sterile needle was used to pick a loopful of a 24 hrs. old culture and was stabbed onto nutrient agar in glass vials. The vials were incubated at 37°C for 24-48 hrs. non-motile bacteria had growth confined to the stab line with definite margins without spreading to surroundings area while motile bacteria gave diffused growth extending from the surface. A positive motility test was indicated by red turbid area extending away from the line of inoculation. A negative test was indicated by red growth along the inoculation line.
- Catalase Test: A small amount of organism was collected from a well-isolated 18-24 hrs. colony with sterile inoculating loop & placed onto the slide. A drop of H2O2 was applied onto the organism on the slide by using a dropper. Observed immediately for bubble formation indicates the presence of catalase enzyme.
- Oxidase test: A piece of filter paper was soaked with few drops of oxidase reagent. Sterile inoculating loop was used to pick a colony of the test organism and smeared on the filter paper. If the organism is oxidase producing, the phenylenediamine in the reagent will be oxidized to a deep purple color.
- Urease Test: The surface of a urea agar slant was streak with a portion of a well-isolated colony from an overnight brain-heart infusion broth culture. The cap was leave loosely and the tube was incubated at 35-37°C in ambient air for 48 hours to 7 days. Examined the development of a pink color for as long as 7 days.
- Hydrogen Sulphide Test: SIM agar slant was prepared. The organism was inoculated into labeled tube by means of stab inoculation. The inoculated tubes were incubated at 37°C for 24-48 hours. The formation of black precipitate on the medium was observed.
- Casein Hydrolysis Test: Nutrient agar plates were prepared with 1% casein. The organism was inoculated on the plate. The plates were incubated at 37°C for 24-48 hours. The agar plates for the presence or absence of a clear zone of proteolysis, surrounding the growth of each of the bacterial test organism was examined.
- Sugar Fermentation: Sugar fermentation test was carried out to determine the ability of organisms to ferment sugars with production of acid and gas.
Carbohydrate fermentation broth was prepared using peptone water medium containing 1% fermentable sugar and 0.01% phenol red. About 10ml of sugar broth was dispensed into each of the test tubes, Durham’s tube which would trap the gas if produced was inverted carefully.
The test tubes were autoclaved and inoculated with a loopful of 24 hrs. old culture of the test organisms after then incubated for 2-7 days at 37°C and observed daily for acid and gas production. Change in color indicates acid production while gas production was indicated by displacement of the medium in the Durham’s tubes.
9. Gelatin Hydrolysis Test: Gelatin agar medium was prepared & sterilized in autoclave for 121°C for 15 mins. Gelatin agar slant was prepared. The gelatin deep were inoculated with 4 to 5 drops of a 24-hour broth culture. Incubation was done at 35°-37°C in ambient air for up to 7-14 days.
The gelatin tube was removed daily from the incubator and place at 4°C to check for liquefaction. (Note: Do not invert or tip the tube, because sometimes the only discernible liquefaction occurs at the top of the deep where inoculation occurred.) An un-inoculated control was refrigerated along with the inoculated tube. Liquefaction is determined only after the control had hardened (gelled).
10. Indole Test: 5 mL of Tryptone broth was placed into different test tubes after which a loopful of the isolated bacterial isolates was inoculated into the test tubes, leaving one of the test tubes uninoculated to serve as control. The test tubes were then incubated at 37°C for 48 hrs.
After incubation, 0.5 mL of Kovac’s reagent was added and shaken gently; it was allowed to stand for 20 min to permit the reagent to rise. A red or red-violet color at the top of the tube indicates a positive result while yellow coloration indicates a negative result.
11. Methyl Red (MR) Test: 5 ml of glucose phosphate broth (1 g glucose, 0.5% KH2PO4, 0.5% peptone and 100 mL distilled water) were dispensed into clean test tubes and sterilized. The tubes were then inoculated with the isolated test organisms and incubated at 37°C for 48 hrs. After incubation few drops of methyl red solution were added to each test and color change was observed. A red color indicates a positive reaction.
12. Voges-Proskauer (VP) Test: 5 ml of glucose phosphate broth (1 g glucose, 0.5% KH2PO4, 0.5% peptone and 100 mL distilled water) were dispensed in clean test tubes and sterilized.
The tubes were then inoculated with the test organisms and incubated at 37°C for 48 hrs. After incubation, 6% α-naphthol and 6% NaOH were added to about 1 mL of the broth culture of the isolated organisms. A strong red coloration was formed within 30 mins. indicates positive reaction.
III. RESULTS & DISCUSSION
A. Isolation, Screening & Maintenance of Amylase Producing Microorganism
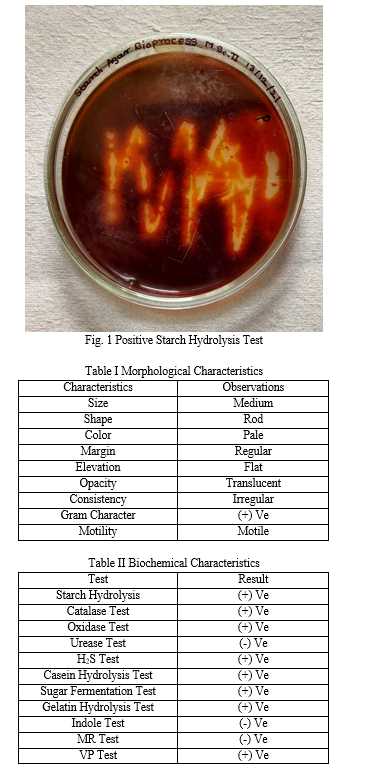
In this study, amylase producing bacterial strains were isolated from the soil. In starch hydrolysis test, zone of clearance with iodine solution was observed.
These colonies were further selected and quantified. Amongst these, the isolate showing maximum absorbance was further optimized and characterized and found to belong to the genus Bacillus.
V. ACKNOWLEDGMENT
We are sincerely grateful to the Department of Biotechnology, Padmashri Vikhe Patil College of Arts, Science & Commerce, Pravaranagar, Maharashtra, India for allowing us to use all facilities for our work, and their encouragement and support.
Conclusion
According to an earlier investigation, the presence of amylolytic organisms from the soil confirms that it is a rich reservoir of amylase makers. The most prevalent manufacturers of enzymes are various types of bacteria, fungus, and actinomycetes. Bacillus species are the most well-known amylase producers. The current study demonstrates the ability of soil microbes to manufacture a very valuable enzyme capable of degrading starch.
References
[1] Oyeleke, S. B. and Oduwole, A. A. (2009): Production of amylase by bacteria isolated from a cassava dumpsite in minna, Nigerstate, Nigeria. Afr. J. Microbiol. Res., 3(4): 143-146. [2] Burhan, A., Nisa, U., Gokhan, C., Ashabil, A. and Osmair, G. (2003): Enzymatic properties of a novel thermostable thermophilic alkaline and chelator resistant amylase from an alkaphilic Bacillus sp. isolate ANT-6. Proc. Biochem., (38): 1397–1403. [3] Bernfeld, P. (1955): Amylases alpha and beta. Methods Enzymol., 1:140-146. [4] Boyer E.W. and Ingle, M. B. (1972): Extracellular alkaline amylase from Bacillus sp. J. Bacteriol., 110: 992-1000. [5] Shaw, J.F., Lin, F.P., Chen, S.C. and Chen, H.C., 1995. Purification and properties of an extracellular -amylase from Thermus sp. Botanical Bulletin of Academia Sinica,36, 195-200. [6] Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003). Microbial ?-amylases: a biotechnological perspective. Process Biochem. 38: 1599-1616. [7] Bernfeld P (1955). Amylase ?and ?, In Methods in Enzymology (Colowick SP, Kaplan NO, ed.), Academic Press Inc, New York 1: 149-158. [8] Miller, G.L. (1959), Use of dinitrosalicyIic acid reagent for determination of reducing sugar. Analytical Chemistry 31 (3), 426-428. [9] Fossi, B.T., F. Taveaand and T. Ndjonenkeu, 2005. Production and partial characterization of a themostable amylase from ascomycetes yeast strain isolated from starchy soils. Afr. J. Biotechnol., 4: 14–18. [10] Jamil et al. \\\\\\\"Isolation of Bacillus subtilis MH-4 from Soil and its Potential of Polypeptidic Antibiotic Production\\\\\\\". Pak J Pharm Sci. January (2007); 20(1):26-31. [11] Stanburg PF, Whitaker A, Hall SJ. (1995) Principles of fermentation Technology (2nd edn). Butterworth Heinemann ltd. (2010); Oxford, U.K. [12] Suman. S, Ramesh K. Production of a thermostable extracellular amylase from thermophilic Bacillus species. J. Pharm. Sci. & Res. (2010); Vol. 2(2), 149-54. [13] Madhav, K., Verma, S. and Tanta (2011). Isolation of amylase producing Bacillus species, from soil sample of different regions in Dehradun and to check the effect of pH and temperatures on their amylase activity. J. Pharm. Biomed. Sci., 12 (03): 8. [14] Pandey, A., Nigam, P., Soccol, C. R., Soccol, V. T., Singh, D. and Mohan, R. (2000): Advances in microbial amylases. Biotechnol. Appl. Biochem., 31: 135- 152. [15] Luang-In V, Yotchaisarn M, Saengha W, Udomwong P, Deeseenthum S, Maneewan K. Isolation and Identification of Amylase-producing Bacteria from Soil in Nasinuan Community Forest, Maha Sarakham, Thailand. Biomed Pharmacol J 2019;12(3).
Copyright
Copyright © 2022 Avinash Gadhave, Mahesh S Wagh, Pravin Kale, Sagar Bhakare. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET46121
Publish Date : 2022-08-01
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

