Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
A Comparative Analysis on the Doses of Covaxin and Covishield Vaccines Administered in India
Authors: Uma Bhattacharya, Mohit Nain
DOI Link: https://doi.org/10.22214/ijraset.2022.41654
Certificate: View Certificate
Abstract
The world has been struggling with the outbreak of Covid- 19 virus for past 21 months. It is a severe acute respiratory syndrome caused by a highly transmissible novel coronavirus. Every country, including India adopted certain measures to combat the pandemic, like announcing lockdown in phases. India has been severely struck by the Covid pandemic. In fact, the third wave of Covid has already knocked in. The development of vaccines against the Covid virus helped the world heal. Different countries are testing the Covid vaccine candidates. The world’s largest vaccination drive started in India on January 16, 2021. The Indian Government has taken a great step forward to vaccinate every individual along with manufacturing the Covid vaccine domestically. The Government of India has also taken effective measures to launch a digital platform Aarogya Setu for the vaccine registration and obtaining the proof that the individual has been vaccinated with the name of the vaccine given and the date on which he/she got vaccinated. In this paper, we have studied the use of Covaxin and Covishield doses in different states of India and found that people who received both doses of the Covid vaccination, whether Covishield or Covaxin, are safe against the Covid virus, according to the government, but they must follow the covid protocols for best outcomes.
Introduction
I. INTRODUCTION
The first coronavirus case was discovered two years ago, and the entire globe has been in a state of terror since then. The coronavirus is thought to have originated in the city of Wuhan, China, with the first case recorded in December 2019. The COVID-19 virus quickly spread over the world. On January 30, 2020, the World Health Organization (WHO) declared the outbreak a Public Health Emergency of International Concern, and on March 11, 2020, it was declared a pandemic (Supady, 2020). In India, the first case of COVID was discovered in Kerala in January 2020 (Brijesh Sathian, 2020). The number of new cases in India increased from 88,600 to 88,600 in just nine months, with a week average of 84,559. The initial wave of the Corona virus was discovered to have a greater impact on the health of the elderly. With their own processes, all countries proclaimed a lockdown. The world came to a halt. The second wave of the Corona virus swept the world soon after, and this strain was found to be lethal. It was discovered that the virus attacked children and adults more aggressively. At first, the aim was to slow down the speed of spreading of the virus. Later, when the cases started to rise enormously and no particular symptoms were available, it became an urgent need for the development of the vaccine. To prevent the virus from spreading, those who were infected were isolated for at least 14 days and were kept in complete quarantine. The Covi-19 virus is very contagious and can strike at any time. The world had never been prepared for a pandemic of this magnitude, and we were racing to produce a vaccine to stop it from spreading. The world appears to have gone insane in its search for a treatment for the coronavirus, an extremely hazardous disease that has engulfed the planet in panic. Medical researchers must produce a vaccine that will provide acquired immunity against the coronavirus 2 (SARS-CoV-2) that causes severe acute respiratory illness.
The first COVID vaccination, the Pfizer-BioNTech COVID-19 vaccine, was licenced by the US Food and Drug Administration for people aged 16 and up. Countries such as China, Russia, and the United States of America,
for instance. On January 16, 2021, India launched the world's largest vaccination campaign. Considering India's large population, importing vaccines would have been prohibitively expensive. Furthermore, because the Indian government wanted to vaccinate every citizen, it was ideal to manufacture the COVID-19 vaccines in India. India also planned to export vaccines to countries that couldn't afford the more expensive vaccines. The goal of manufacturing COVID vaccines in India is to ensure that each citizen in the country receives all of the required doses, as well as to make them affordable to the world's poorest countries. Covishield and Covaxin are mostly used in India at the moment. There were also rumours about the COVID vaccinations' ineffectiveness. Some of them include the following: they are unsafe, they induce infertility, they modify DNA, they are unsafe for persons with allergies, vaccines are not thoroughly evaluated, and so on. All of these, however, turned out to be rumours.
All of the COVID-19 vaccines that have been licenced for use have been thoroughly evaluated, are entirely safe, and appropriately trigger the COVID virus, according to studies. Furthermore, the race to develop a powerful cure (vaccine) for the sickness drove them to develop and release the most effective vaccine, which might potentially trigger the symptoms of any future form of the Corona Virus.
II. RELATED WORKS
Ever since the SARS-CoV2 Virus has struck the world, there is a constant urge and interest of people to know, to find out and research about it. What is this Corona Virus? From where and how did this even originated? Why is this virus so dangerous to people? How is the world handling it? What precautions do we need to take to curb its growth? Is there a requirement for a vaccine? If yes, will the vaccine be able to provide shield to us from the virus? All these questions led people and researchers to dive into this topic and bring into light the cause, effect and remedies of this SARS-CoV 2. V.M. Kumar et al. (2021) in Strategy for COVID-19 vaccination in India: the country with the second highest population and number of cases, has vividly described each and every Covid Vaccine manufactured in India, explained their trial phase alongwith the strategy adopted by India for Covid-19 vaccination (Velayudhan Mohan Kumar, 2021). H. Dai et al. (2020) in their editorial who is running faster, the virus or the vaccine? has described about the pros and cons of the virus as well as the vaccine. They have explained how the pace of people being vaccinated is getting affected. Also, what measures are taken for vaccinating the mass (Han Dai, 2020). J. Connor et al. (2020) in their review article Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: A review has brought out the risk factors that affected women during the Covid pandemic. The article concluded that Gender differences in health risks and implications are likely to be expanded during the Covid-19 pandemic (Jade Connor, 2020). J. Muthukrishnan et al. (2021) has done a cross sectional study based on a designated COVID hospital in New Delhi. This study was done on all the patients admitted to the hospital having moderate to severe COVID-19 symptoms. They fitted a logistic regression model to the data and concluded that people with complete vaccination status and of younger age were associated with survival (Vaccination status and COVID-19 related mortality: A hospital based cross sectional study, 2021). Samaddar et al. in their paper (The Enigma of Low COVID-19 Fatality Rate in India, 2020) has talked about how the Indian SARS-CoV-2 Strains differ from the strains elsewhere. They discussed the mutations and does variations in host factors determine the susceptibility to sars-cov-2. Sharma et al. (2020) and Wang et al. (2020) has discussed about the challenges that came through in the development of Covid vaccine. They studied about the immune response to SARS-COV2 virus. Then, various clinical trials and phases of vaccine development and their various platforms like DNA and RNA based vaccines, non-replicating viral vector vaccines, inactivated vaccines, etc. (A Review of the Progress and Challenges of Developing a Vaccine for COVID-19, 2020) (The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation, 2020). We wanted to study how the COVID-19 vaccination drives throughout India was carried out. Our primary goal is to investigate how the two COVID-19 vaccines now in use in India, Covishield and Covaxin, affect the survival rate. Another goal of this research is to determine which of the two COVID vaccines is superior in terms of providing robust immunity and having the least negative impact on people's health.
III. MATERIALS AND METHODS
There are three types of data in the dataset used for this investigation. The initial set of data pertains to COVID-19 cases that have been detected, cured, and died across India's states and union territories. It takes place between January 30, 2020, and August 11, 2021. The second type of information is state-by-state testing information for COVID-19 cases found, including the number of positive and negative cases. From April 17, 2020, to August 10, 2021, it was recorded. The third kind has the combined data of Covid-19 vaccinations, which distinguishes between Covishield and Covaxin doses. The dataset is also segmented by the Indian government's age-group slabs. From January 16, 2021, to August 9, 2021, it was recorded. This entire dataset was taken from Kaggle's official website (https://www.kaggle.com/) (https://www.kaggle.com/sudalairajkumar/covid19-in-india). Tableau and MS Excel were used to conduct the analysis. The dataset consists 7643 rows and 24 columns.
The following are the fields in the dataset:
- Updated On: This column contains information about the date starting from 16-01-2021 to 15-08-2021 with no gaps.
- State: This column contains all the States and Union Territories of India in Alphabetical order starting from Andaman and Nicobar Islands to West Bengal.
- Total Doses Administered: This column contains information on the total number of doses administered in each state and union territory of India.
- Sessions: This column contains information about the number of sessions that are held in each state and union territory.
- Sites: This column contains numeric information about the number of sites formed for vaccination campaign in the particular state.
- First Dose Administered: This column tells the number of first doses of vaccines administered in the state on the given date.
- Second Dose Administered: This column tells the number of second doses of vaccines administered in the state on the given date.
- Male (Dose Administered): It contains information about the number of doses administered among which Males were administer.
- Female (Dose Administered): It contains information about the number of doses administered among which Females were administer.
- Transgender (Dose Administered): It contains information about the number of doses administered among which Transgender were administer.
- Covaxin (Dose Administered): It contains information about the number of Covaxin doses administered in the State/UT.
- Covishield (Dose Administered): It contains information about the number of Covishield doses administered in the State/UT.
- Sputnik V (Dose Administered): It contains information about the number of Sputnik V doses administered in the State/UT.
- AEFI: It tells us about an adverse event following immunization (AEFI). It is any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine.
- 18-44 Years (Dose Administered): It contains information about the number of doses administered for the age group 18-44 years in the State/UT.
- 45-60 Years (Dose Administered): It contains information about the number of doses administered for the age group 45-60 years in the State/UT.
- 60 + Years (Dose Administered): It contains information about the number of doses administered for the age group 60+ years in the State/UT.
- 18-44 Years (Individuals Vaccinated): It contains information about the number of Individuals vaccinated in the age group 18-44 years in the State/UT on the particular date.
- 45-60 Years (Individuals Vaccinated): It contains information about the number of Individuals vaccinated in the age group 45-60 years in the State/UT on the particular date.
- 60+ Years (Individuals Vaccinated): It contains information about the number of Individuals vaccinated in the age group 60+ years in the State/UT on the particular date.
- Male (Individuals Vaccinated): It contains information about the number of Male Individuals vaccinated in the State/UT on the particular date.
- Female (Individuals Vaccinated): It contains information about the number of Female Individuals vaccinated in the State/UT on the particular date.
- Transgender (Individuals Vaccinated): It contains information about the number of Transgender Individuals vaccinated in the State/UT on the particular date.
- Total Individuals Vaccinated: It contains information about the Total number of Individuals vaccinated in the State/UT on the particular date.
IV. COVID VACCINATION IN INDIA
The Indian government took significant measures to respond to the worldwide pandemic and began preparing the health system to handle all elements of COVID-19 management. As a result, India was able to maintain the lowest mortality rate and the highest recovery rate in the world during the pandemic (www.mohfw.gov.in, 2021). On January 16, 2021, free immunisation against SARS-CoV-2 began. Vaccinating a large portion of the population during the COVID epidemic and its variants was a big task for the Indian government. According to the most recent statistics, India has a population of 1.39 billion people. As a result, mass vaccination faced a significant challenge, but the current government's management was able to overcome it. The following vaccinations are produced in India:
- Covaxin by Bharat Biotech International Limited in collaboration with National Institute of Virology of ICMR. It is the first domestic covid vaccine manufactured in India.
- Covishield by Serum Institute of India (Pune).
- ZyCoV-D by Cadila Healthcare (Ahmedabad)
- Sputnik-V by Dr. Reddy’s Laboratories (Hyderabad), originally developed by Gamaleya National Center of Epidemiology and Microbiology of Moscow, Russia.
- mRNA vaccine by Gennova Biopharmaceuticals Ltd (Pune) in collaboration with HDT Biotech Corporation (USA).
The Indian government established the NEGVAC (National Expert Group on Vaccine Administration) for COVID-19. It was designed specifically to provide guidelines covering all aspects for the Covid Vaccine Administration in India. According to NEGVAC, it was determined that the COVID vaccines would be distributed initially to frontline workers, health personnel, and those over the age of 60. Several centres were established around India to make the vaccine more accessible. Vaccines were next made available to adults between the ages of 45 and 60, and then to those between the ages of 18 and 44.
V. EXPLORATORY DATA ANALYSIS
Following are the analysis of the dataset taken for this study using the software Tableau & MS Excel.
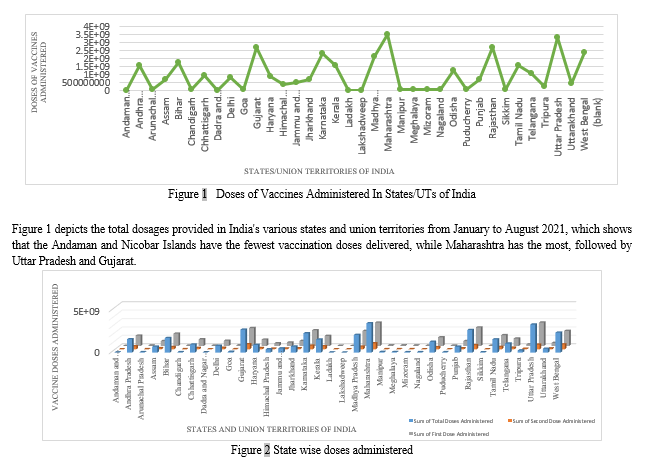
Figure 2 shows a 3-D representation of the state-by-state distribution of total doses separated into first and second doses in India from January to August 2021. The sum of total doses administered in the particular state or UT is represented by the blue bar; the sum of the first dose administered in the particular state or UT is represented by the grey bar; and the sum of the second dose administered in the particular state or UT is represented by the orange bar. This graph depicts the total number of immunizations given vs. the first and second doses.
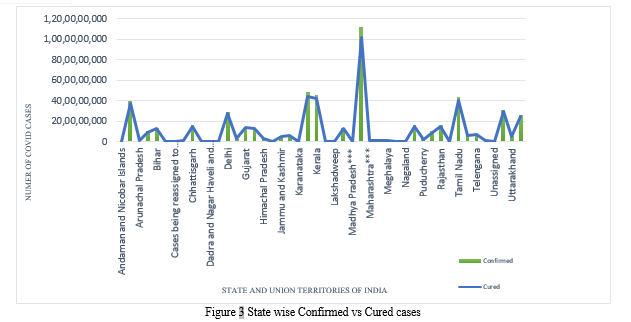
Figure 3 provides a graphical representation of confirmed Covid cases and cured cases among those confirmed patients in various Indian states, with data spanning January to August 2021. A clustered column graph and a line graph are combined in Figure-3. The green-colored clustered column graph depicts confirmed Covid Positive cases, while the blue-colored line graph depicts the Cured Covid Patients in different Indian states and union territories. Maharashtra has the largest confirmed number of COVID patients as well as the greatest number of COVID cases that have been cured. With the use of Treemaps, this figure is further explained below.
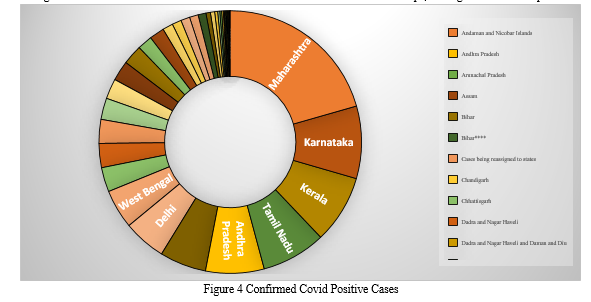
Figure 4 shows a tree map of confirmed COVID-19 positive cases from various Indian states and union territories. A tree map is a way for showing hierarchical data that uses nested objects, usually rectangles, to display it. We can see that Maharashtra has the most verified COVID cases, with 19, followed by Karnataka, Uttar Pradesh, and so on, by comparing the sizes of the rectangles. Similarly, the number of verified COVID positive cases is lowest in Daman and Diu.
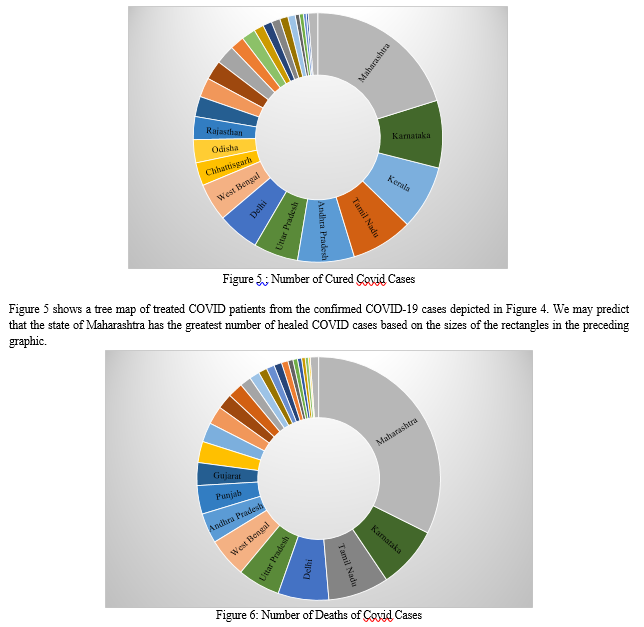
Figure 6 depicts the number of deaths caused by Covid 19 among the confirmed cases of Covid 19 in India's various states and union territories. Based on the diameters of the rectangles, we may deduce that Maharashtra has the largest number of deaths among the confirmed covid positive cases in India.
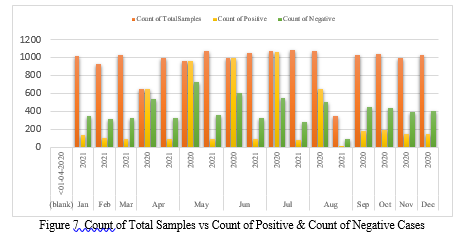
In Figure 7, the orange bar represents the total number of samples collected, the yellow bar represents the number of positive instances, and the green bar represents the number of negative cases from April 2020 to August 2021. It depicts a comparison study that compares the number of total samples to the number of positive and negative cases. According to the graph above, the number of positive instances is largest in July 2020 and lowest in August 2021, whereas the number of negative cases is highest in May 2020 and lowest in August 2021. Furthermore, we can see that the number of positive cases in 2021 is lower than in 2020 due to the Covid Vaccination Drive. Table 1 contains a tabular representation of Figure 4 that provides a detailed perspective of the above graphical representation.
|
Row Labels |
Count of Total Samples |
Count of Negative |
Count of Positive |
|
Andaman and Nicobar Islands |
453 |
1 |
430 |
|
2020 |
232 |
1 |
231 |
|
2021 |
221 |
199 |
|
|
Andhra Pradesh |
488 |
436 |
123 |
|
2020 |
266 |
237 |
123 |
|
2021 |
222 |
199 |
|
|
Arunachal Pradesh |
477 |
428 |
116 |
|
2020 |
255 |
254 |
116 |
|
2021 |
222 |
174 |
|
|
Assam |
469 |
48 |
114 |
|
2020 |
247 |
48 |
114 |
|
2021 |
222 |
||
|
Bihar |
489 |
1 |
126 |
|
2020 |
267 |
1 |
126 |
|
2021 |
222 |
||
|
Chandigarh |
479 |
478 |
127 |
|
2020 |
264 |
264 |
127 |
|
2021 |
215 |
214 |
|
|
Chhattisgarh |
482 |
63 |
126 |
|
2020 |
260 |
63 |
126 |
|
2021 |
222 |
||
|
Dadra and Nagar Haveli and Daman and Diu |
170 |
170 |
151 |
|
2020 |
170 |
170 |
151 |
|
Delhi |
489 |
22 |
123 |
|
2020 |
267 |
22 |
123 |
|
2021 |
222 |
||
|
Goa |
483 |
2 |
125 |
|
2020 |
261 |
2 |
125 |
|
2021 |
222 |
||
|
Gujarat |
487 |
95 |
171 |
|
2020 |
267 |
95 |
171 |
|
2021 |
220 |
||
|
Haryana |
492 |
341 |
148 |
|
2020 |
270 |
270 |
147 |
|
2021 |
222 |
71 |
1 |
|
Himachal Pradesh |
488 |
487 |
128 |
|
2020 |
266 |
266 |
128 |
|
2021 |
222 |
221 |
|
|
Jammu and Kashmir |
489 |
489 |
129 |
|
2020 |
267 |
267 |
129 |
|
2021 |
222 |
222 |
|
|
Jharkhand |
484 |
483 |
416 |
|
2020 |
263 |
263 |
220 |
|
2021 |
221 |
220 |
196 |
|
Karnataka |
491 |
108 |
131 |
|
2020 |
269 |
108 |
131 |
|
2021 |
222 |
||
|
Kerala |
497 |
73 |
307 |
|
2020 |
275 |
73 |
275 |
|
2021 |
222 |
32 |
|
|
Ladakh |
294 |
249 |
93 |
|
2020 |
175 |
161 |
93 |
|
2021 |
119 |
88 |
|
|
Lakshadweep |
195 |
||
|
2021 |
195 |
||
|
Madhya Pradesh |
492 |
411 |
140 |
|
2020 |
270 |
270 |
130 |
|
2021 |
222 |
141 |
10 |
|
Maharashtra |
488 |
195 |
196 |
|
2020 |
266 |
195 |
196 |
|
2021 |
222 |
||
|
Manipur |
406 |
80 |
|
|
2020 |
193 |
80 |
|
|
2021 |
213 |
||
|
Meghalaya |
409 |
346 |
106 |
|
2020 |
212 |
150 |
106 |
|
2021 |
197 |
196 |
|
|
Mizoram |
465 |
2 |
125 |
|
2020 |
260 |
2 |
125 |
|
2021 |
205 |
||
|
Nagaland |
484 |
69 |
130 |
|
2020 |
264 |
69 |
130 |
|
2021 |
220 |
||
|
Odisha |
492 |
13 |
142 |
|
2020 |
270 |
13 |
142 |
|
2021 |
222 |
||
|
Puducherry |
478 |
464 |
276 |
|
2020 |
257 |
257 |
223 |
|
2021 |
221 |
207 |
53 |
|
Punjab |
491 |
51 |
132 |
|
2020 |
269 |
51 |
132 |
|
2021 |
222 |
||
|
Rajasthan |
491 |
263 |
134 |
|
2020 |
269 |
263 |
134 |
|
2021 |
222 |
||
|
Sikkim |
413 |
64 |
93 |
|
2020 |
229 |
64 |
93 |
|
2021 |
184 |
||
|
Tamil Nadu |
491 |
83 |
135 |
|
2020 |
269 |
83 |
135 |
|
2021 |
222 |
||
|
Telangana |
419 |
30 |
75 |
|
2020 |
200 |
30 |
75 |
|
2021 |
219 |
||
|
Tripura |
447 |
444 |
431 |
|
2020 |
248 |
245 |
237 |
|
2021 |
199 |
199 |
194 |
|
Uttar Pradesh |
490 |
69 |
121 |
|
2020 |
268 |
68 |
121 |
|
2021 |
222 |
1 |
|
|
Uttarakhand |
491 |
490 |
132 |
|
2020 |
269 |
269 |
132 |
|
2021 |
222 |
221 |
|
|
West Bengal |
493 |
1 |
130 |
|
2020 |
271 |
1 |
130 |
|
2021 |
222 |
||
|
(blank) |
|||
|
<01-04-2020 |
|||
|
Grand Total |
16336 |
6969 |
5662 |
Table 1 Tabular Representation of Figure 5
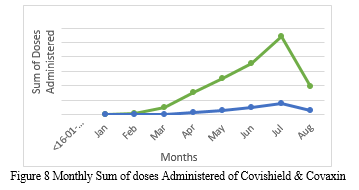
Figure 8 displays the monthly sum of Covaxin and Covishield dosages given from January to August 2021. The green line graph indicates the total number of Covishield vaccine doses administered, whereas the blue line graph depicts the total number of Covaxin doses administered. It is a comparison of the two doses provided. The aggregate of each dose grows from January to July, then declines in August. The figure above shows that the total number of Covishield vaccine doses administered is more than the total number of Covaxin doses administered. Furthermore, we can forecast that the month of July would have the highest total of all doses provided.
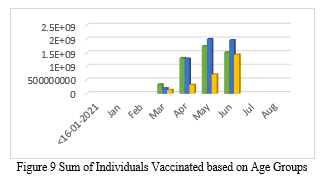
The Government of India announced age slabs for the smooth movement of vaccination drive. The age slabs were 60+ Years, 45-60 Years, and 18-44 Years. Based on this, Figure 6 shows the graphical representation of individuals vaccinated from January 2021 to August 2021. The Green bar shows vaccination of individuals aged 60+ years; Blue bar shows vaccination of individuals aged 45-60 years; and the yellow bar shows the vaccination of individuals aged 8-44 years. We can see from the representation that maximum number of individuals were vaccinated in the month of June. If we look deeper into the representation, we can see that the maximum number of individuals vaccinated are of the age group 45- 60. One point to note here is that, since the vaccination drive in India started in phases and that the vaccination of individuals aged between 18-44 years were at the last, the number of individuals vaccinated is therefore less.
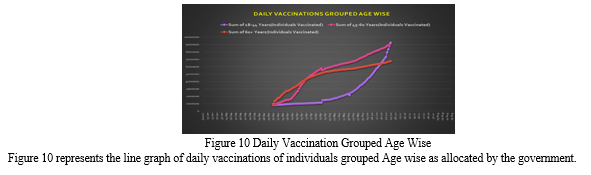
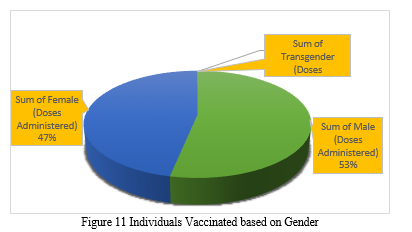
Figure 11 illustrates the total number of people vaccinated and differentiated by gender, i.e., male and female, from January 2021 to August 2021with the help of a pie chart. We can infer from the preceding figure that the number of males vaccinated is greater than the number of females vaccinated, i.e. 53% Males have been vaccinated while 47% Females got vaccinated according to the data used.
VI. DISCUSSION
Implementing mass vaccination in a country with a large population, such as India, is undoubtedly a difficult assignment for the government. India, on the other hand, was able to overcome the obstacle by efficiently managing the immunisation drives. The federal government directed those states begin teaching worried healthcare workers in the correct use of vaccines. Groups were constituted and established for the scientific guidance on the vaccination programme. National Expert Group on Vaccine Administration for COVID-19 (NEGVAC) was constituted in August 2020, to formulate comprehensive action plan for vaccine administration (2021). National Task Force for Focused Research on Corona Vaccine was established in April 2020 to guide the process for coronavirus vaccine development, encourage domestic Research & Development of Drugs, Diagnostics and Vaccines. An Empowered Group on Vaccine Administration for COVID-19 (EGVAC) has also been constituted in January 2021, to facilitate optimal utilization of technology so as to make COVID vaccination all inclusive, transparent, simple and scalable, headed by CEO, National Health Authority. National Technical Advisory Group on Immunization (NTAGI), through its Standing Technical Sub-Committee (STSC) and COVID-19 Working Group, has been providing advice on technical matters with respect to COVID-19 vaccination (mohfw.gov.in, 2020). The National Informatics centre under the Ministry of Electronics and Information Technology developed a mobile application named Aarogya Setu on 6 July 2020. It is an Indian COVID-19 contact – tracing, syndromic mapping and self-assessment digital service app. This app is available in 12 different languages. It is a tracking app which takes into account the GPS and Bluetooth features of ones device to determine the risk if one has been within six feet of a Covid-19-infected person, by scanning through a database of known cases across India. Using location information, it determines whether the location one is in belongs to one of the infected areas based on the data available. The app crossed 5 million installations within three days of its launch. It was revealed that this app has been able to identify more than 3,000 hotspots in 3-17 days ahead of time. Similarly , the Co-Win Software is a one-of-a-kind digital app that allows individuals to apply online for vaccine slots using their mobile number and unique ID such as Aadhar No., Voter ID, and so on, and book the closest place accessible to them without having to drive far to obtain the vaccine. It is an Indian government web portal for Covid-19 vaccination registration, owned and operated by India’s Ministry of Health and Family Welfare. It is available in 12 different languages. It was launched on 16 January 2021. The platform has been integrated in the Aarogya Setu App. After successfully receiving the vaccine, the individual could download the vaccination certificate, which included information such as the name of the vaccine taker, the name of the vaccine giver, the location and date on which the individual received the vaccine dose (first/second), and the date on which the individual is eligible to receive the next dose.
As of now, three vaccines can be registered on the platform Covishield, Covaxin and Sputnik V. Currently, two doses of each vaccine, Covaxin and Covishield, are administered. Individuals aged 18 and up are successfully receiving free vaccinations at various vaccination centres. Trials for vaccine candidates for people under the age of 18 are currently underway, so that children under the age of 18 can receive the vaccination without losing their immunity.
VII. COMPETING INTERESTS
The authors declare that there are no competing interests.
VIII. AUTHOR CONTRIBUTION
Both the authors conceived of the presented idea. U.B. developed the theory and performed the computations. U.B. and M.N. verified the analytical methods. M.N. encouraged U.B. and supervised the findings of this work. Both the authors discussed the results and contributed to the final manuscript. M.N. provided critical feedback and helped shape the research, analysis and manuscript.
XI. CONFLICT OF INTEREST
This article does not contain any studies with human participants or animals performed by any of the authors.
References
[1] Wang, J., Peng, Y., Xu, H., Cui, Z. and Williams, R., 2020. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech, 21(6).
[2] Dai, H., Han, J. and Lichtfouse, E., 2020. Who is running faster, the virus or the vaccine?. Environmental Chemistry Letters, 18(6), pp.1761-1766.
[3] Forni, G. and Mantovani, A., 2021. COVID-19 vaccines: where we stand and challenges ahead. Cell Death & Differentiation, 28(2), pp.626-639.
[4] Kumar, V., Pandi-Perumal, S., Trakht, I. and Thyagarajan, S., 2021. Strategy for COVID-19 vaccination in India: the country with the second highest population and number of cases. npj Vaccines, 6(1).
[5] Sharma, O., Sultan, A., Ding, H. and Triggle, C., 2020. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Frontiers in Immunology, 11.
[6] Samaddar, A., Gadepalli, R., Nag, V. and Misra, S., 2020. The Enigma of Low COVID-19 Fatality Rate in India. Frontiers in Genetics, 11.
[7] Kaplan, R. and Milstein, A., 2021. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proceedings of the National Academy of Sciences, 118(10).
[8] Ramatillah, D. and Isnaini, S., 2021. Treatment profiles and clinical outcomes of COVID-19 patients at private hospital in Jakarta. PLOS ONE, 16(4), p.e0250147.
[9] El-Elimat, T., AbuAlSamen, M., Almomani, B., Al-Sawalha, N. and Alali, F., 2021. Acceptance and attitudes toward COVID-19 vaccines: A cross-sectional study from Jordan. PLOS ONE, 16(4), p.e0250555.
[10] Florindo, H., Kleiner, R., Vaskovich-Koubi, D., Acúrcio, R., Carreira, B., Yeini, E., Tiram, G., Liubomirski, Y. and Satchi-Fainaro, R., 2020. Immune-mediated approaches against COVID-19. Nature Nanotechnology, 15(8), pp.1-16.
[11] Mohfw.gov.in. 2022. The World\'s Largest VAACCINATION Drive. [online] Available at:
Copyright
Copyright © 2022 Uma Bhattacharya, Mohit Nain . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET41654
Publish Date : 2022-04-20
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

