Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Formulation and In- Vitro Evaluation of Baclofen Gastroretentive Microballoons for Sustained Drug Delivery
Authors: J. Gomathi, T. Muthukumaran, Kavitha. C, Priyanka Sinha, Sabbathyan Balla
DOI Link: https://doi.org/10.22214/ijraset.2022.46820
Certificate: View Certificate
Abstract
A sustained release drug delivery system for baclofen was designed to increase its residence time in the stomach without establishing contact with the mucosa. This was made possible through the preparation of microballoons by emulsion solvent diffusion-evaporation technique. The prepared microballoons were characterized for physical characteristics such as particle size, particle shape and surface morphology by scanning electron microscopy. Further, percentage yield determination, drug entrapment efficiency, in vitro buoyancy test, micromeritic investigations and in vitro drug release studies were carried out. The obtained microspheres were free flowing, spherical and displayed a particle size ranging from 50.15 to 67.0µm suitable for oral delivery. The drug entrapped in the hollow microspheres increased with the increase in eudragit RSPO content. Scanning electron microscopy and particle size analysis revealed differences in the formulations in respect to their appearance and size distribution. The FTIR spectroscopy technique and DSC were carried out to rule out drug – excipients interactions. From the results obtained, it was concluded that there was no interaction between drug and the excipients. The formulation containing Baclofen:Eudragit RSPO (1:3 and 1:4) exhibited higher percentage values for buoyancy time. The drug release was found to follow Higuchi kinetics with non-fickian diffusion mechanism, from all the four batches. These preliminary results indicate that baclofen loaded microballoons could be effective in sustaining drug release for prolonged periods of time.
Introduction
I. INTRODUCTION
The oral drug administration is the most predominant route for drug delivery due to ease of dosing administration, patient compliance, and flexibility in formulation. Effective oral drug delivery process depends on the factors such as gastric emptying process, gastrointestinal transit time of dosage form, drug release from the dosage form, and site of absorption of drugs. Oral controlled release drug deliveries have recently been of increasing interest in the pharmaceutical field to achieve improved therapeutic advantages. It is primarily aimed at achieving more predictable to control the drug release, and increased bioavailability, thereby obtaining a maximum therapeutic effect [1].
Gastroretentive dosage forms (GRDFs) are a drug delivery formulation that is designed to remain in the gastric region for a long period and significantly prolong the gastric retention time (GRT) of drugs. This technology has generated enormous attention over the last few decades owing to its potential application to improve the oral delivery of some important drugs. GRDFs prolonged retention in the upper gastrointestinal tract, greatly improve their oral bioavailability and/or their therapeutic outcome [2]. Various techniques were used to encourage gastric retention of an oral dosage form. A significant approach for the treatment of gastric disorders can be achieved by floating drug delivery systems (FDDS). FDDS have a bulk density less than gastric fluids; they can float on the gastric juice in the stomach; the drug is released slowly at the desired rate from the system [3]. A number of FDDS involving various technologies have been developed such as single and multiple-unit hydrodynamically balanced systems, single and multiple-unit gas generating systems, hollow microspheres, and raft forming systems [4].
Hollow microspheres/microballoons are considered as one of the most promising buoyant systems. They possess the unique advantages of multiple-unit systems as well as better floating properties because of central hollow space inside the microsphere. The drug release and better-floating properties mainly depend on the type of polymer, plasticizer, and the solvents used for formulation. Commonly used polymers such as polycarbonate, cellulose acetate, calcium alginate, Eudragit S, agar and low methoxylated pectin were used in the preparation of hollow microspheres [5]. Hollow microspheres loaded with drugs in their outer polymer shell were prepared by simple solvent evaporation or solvent diffusion evaporation method to prolong the GRT of the dosage form. The microballoons floated continuously over the surface of an acidic dissolution media containing a surfactant for more than 12 hrs [6].
Baclofen is a gamma-aminobutyric acid (GABA) agonist used as a skeletal muscle relaxant used for the relief of painful and uncomfortable muscle spasms caused by a variety of conditions. It is known to be particularly useful in treating muscle spasticity associated with spinal cord injury. Baclofen is administered for the relief of signs and symptoms of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and associated pain and clonus, in addition to muscular rigidity. Baclofen has a bioavailability of 70% to 85% and is therefore rapidly absorbed through the gastrointestinal tract following oral administration. Peak plasma concentrations are generally observed 2 to 3 hours after ingestion. The absorption is dose-dependent and increases with higher doses. Baclofen is rapidly and extensively absorbed and eliminated. The half-life of the drug is ∼2.5 to 4 hrs in plasma. Baclofen has absorption window in upper Gastrointestinal (G.I.) tract. Baclofen is difficult to formulate in to sustained release dosage forms because on arrival to colon its absorption is diminished or non-existent [7, 8]. In the present investigation efforts were made to formulate floating microspheres of Baclofen to improve the absorption of Baclofen in stomach, to prepare floating microballons, to study sustained effect of floating microballons, to study the effect of different polymers on buoyancy and % drug release and Statistical optimization of factorial design formulation.
II. MATERIALS AND METHODS
A. Materials
Baclofen was kindly gifted by Intas Pharmaceuticals Pvt. Ltd., Ahmedabad, India, Eudragit RSPO used as polymer which was purchased from Ana lab Fine Chemicals (Mumbai, India). Polyvinyl alcohol, Tween 20 and Tween 80 were purchased from Mayuraa chemicals, Chennai, India. All other reagents used were of analytical grade
B. Methods
- Preparation of Microballoons: Baclofen loaded Eudragit RSPO microballoons were prepared by employing emulsion solvent diffusion-evaporation technique [9]. Weighed amount of AH was mixed with Eudragit RSPO (in ratios of 1:1, 1:2, 1:3 and 1:4) in a solvent mixture of dichloromethane and ethanol (1:1) at room temperature. The resulting drug-polymer solution was poured gradually into 200 mL of water containing 0.75% w/v polyvinyl alcohol and the preparation was stirred at 400 rpm for 3h at 400C. The finely formed microballoons were then filtered, washed with water and dried overnight at room temperature. The composition of the formulations is displayed in Table 1.
Table 1: Formulation compositions of Baclofen Loaded Microballoons
|
Formulation code |
Polymer to drug ratio |
Solvent mixture |
Concentration of PVA (%w/v)
|
Stirring speed (rpm)
|
|
MB1 |
1:1 |
1:1 |
0.75 |
400 |
|
MB2 |
2:1 |
1:1 |
0.75 |
400 |
|
MB3 |
3:1 |
1:1 |
0.75 |
400 |
|
MB4 |
4:1 |
1:1 |
0.75 |
400 |
Solvent mixture (Dichlomethane and Ethanol); PVA: Polyvinyl alcohol
C. Characterization of Microballoons
- Micromeritic Properties: The microballoons were characterized by their micromeritic properties such as particle size, Bulk density, tapped density, % compressibility index, and flow properties such as angle of repose [10].
- Particle Size: The particle size analysis of microballoons was determined with an optical microscopic method [11]. The particle size of the prepared microballoons was dispersed in glycerin, and a drop of the above dispersion was transferred on a glass slide and observed under an optical microscope under regular polarized light, and the mean particle size was calculated by measuring 100 microballoons (n=3) with the help of a calibrated eyepiece micrometer and stage micrometer. The average diameter was calculated using the following formula: Average diameter =Total diameter of microballoons / Number of microballoons × Calibration factor
- Bulk density/fluff Density: It is the ratio between a given mass of a powder and its bulk volume. Initial density also is known as fluff or poured bulk density. Bulk density of the formulated microballoons was determined by taking a known quantity about 2 g of formulated microballoons in a clean measuring cylinder, and initial volume was measured. The bulk density was calculated by the following equation: Bulkdensity = Mass of microballoons in grams / Volume of microballoons in cm3
- Tapped Density: Tapped density of microballoons was done by the tapping method [12]. Formulated microballoons (2 g) were transferred into 10 mL measuring cylinder. After observing the initial volume of microballoons, the tapping was continued on a hard surface until no further change in volume was calculated using tapped density apparatus (Pharma Chem Machineries, model C-BD 100). The tapped density was calculated according to the following formula: Tapped density = Mass of Microballons in grams/ volume of microballoons after tapping in cm2
- Compressibility Index: The percentage compressibility of powder is a direct measure of the potential powder arch or bridge strength and stability and also called as Carr’s index and is computed according to the following equation: % Compressibility index = Tapped density-Fluff density/Tapped density × 100
- Angle of Repose: The flow property of floating microballoons is usually assessed by determining the angle of repose. The angle of repose of microballoons was determined by fixed funnel method [13]. The microballoons were allowed to fall freely through a funnel until apex of conical pile just touched the tip of the funnel. The angle of repose ø was determined according to the following formula: Angle of repose = tan-1 Height of the pile (h)/ Radius of base of the pile (r)
D. Evaluation Parameters of Microballoons
- Investigation of drug polymer compatibility Fourier transform Infrared Spectroscopy: (FTIR) of pure drug AH and mixture of AH with Eudragit RSPO were taken using Shimadzu FTIR 8400 spectrophotometer to determine drug polymer compatibility. Samples were prepared with potassium bromide and the spectral data was recorded over a spectral range of 400 to 4500 cm -1.
- Differential Scanning Calorimetry (DSC): DSC (Mettler- 7, Germany) was performed to study the thermal behaviour of drug alone and mixture of drug and polymer. Scans of final formulae were done at 50 to 300°C with a heating range of 10°C/min.
- Percentage Yield of Floating Microballoon: Percentage yield of floating microballoon formulation was determined by weighing after drying [14]. The actual weight of microballoons was divided by the total weight of all the non-volatile components used for the preparation of microballoons and is represented by the following formula: % Yield =Actual weight of floating microballoons / Total weight of Excipients+Drug) X 100
- Determination of drug Entrapment Efficiency: The floating microballoons (10 mg) were taken and dissolved in 10 mL of ethanol and volume was made up with 0.1 N HCl. The solution was filtered and analyzed spectrophotometrically (Shimadzu UV-1700 S Japan) at 266 nm using the calibration curve. Each batch should be examined for drug content in a triplicate manner. The entrapment efficiency of floating microballoons can be calculated by the following formula: Entrapment efficiency=Actual drug content/ Theoretical drug content ×100
- In- vitro buoyancy: Microballoons equivalent to 100 mg were spread over the surface of a USP XXIV Dissolution apparatus (Type II) filled with 900 mL of 0.1 N HCl fluid (pH 1.2) containing Tween 80 (0.02 w/v %) to stimulate gastric fluid at 37°C [16]. The medium was agitated with a paddle rotating at 100 rpm at 37±0.5°C for 12 hrs. After 12 hrs, the floating and settled portions of microballoons were recovered separately. The microballoons were dried and weighed.[15] The buoyancy percentage of microballoons was calculated by the following equation: Buoyancy (%)=Floating microballoons/Total mass of microballoons × 100
- Surface Morphology: The surface morphology of microballoons was investigated using scanning electron microscopy (SEM) [16]. The samples for SEM were prepared by lightly sprinkling the microballoons powder on a double adhesive tape which stuck to a stub. The stubs were then coated with platinum under an argon atmosphere using a gold sputter module in a high vacuum evaporator. The samples were then randomly scanned, and photomicrographs were taken on higher magnification (×500) for surface morphology.
- In- vitro Evaluation of Floating Behavior: Gastroretentivebaclofen microballoons (100 mg) were dispersed in 0.1 N HCl (900 ml, pH 1.2, 37±0.5oC) containing Tween 20 (0.02 w/v %) to assess the in-vitro floating behavior. The medium was agitated by a paddle at 100 rpm (USP 29 dissolution apparatus 1). At predetermined time intervals, the layer of buoyant microballoons was removed and the floating microballoons were recovered by filtration. Microballoons settled at the bottom of the flask were recovered separately by filtration. Both floating and non-floating types were dried at 40oC till constant weight. After drying, each fraction of the microballoons was weighed and the percentage of floating microballoons was calculated by the following equation[17]: % Floating Microballoons = weights of the floating / (weights of the floating + the settled microballoons) × 100
- In- vitro Baclofen Release: The level of baclofen release from microballoons (400- 1100 μm in diameter) was measured by the paddle method (USP 29 dissolution apparatus 1) rotated at 100 rpm (Electrolab, TDT-08L, USA). Microballoons (120 mg) equivalent to single dose of 30 mg drug were dispersed in 0.1 N HCl (900 ml, pH 1.2, 37oC) containing Tween 20 (0.02 w/v%). The level of baclofen release was estimated spectrophotometrically using a UV-detector at 266 nm (UV-160A, Shimadzu, Japan). The dissolution studies were repeated six times in 0.1 N HCl (pH 1.2). The mean values are plotted as percent cumulative release versus time.
III. RESULTS AND DISCUSSION
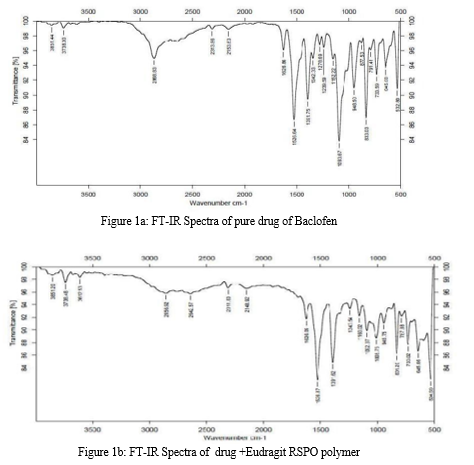
The FT-IR spectra of the drug – polymer mixture and pure baclofen are portrayed in Figure.1. The IR spectra of drug-polymer mixture exhibited distinctive peaks at 3286.8 cm-1 due to OH stretch, 3227.0 c -1 due to NH stretch. The FT-IR spectra of baclofen displayed characteristic peak at 3670 – 3580 cm-1due to OH stretching and at 3389.0 cm-1 due to – to NH stretch. From the above characteristic peaks, it can be noted that there is no significant change in the wave numbers indicating the absence of incompatibility between the drug and the excipients.
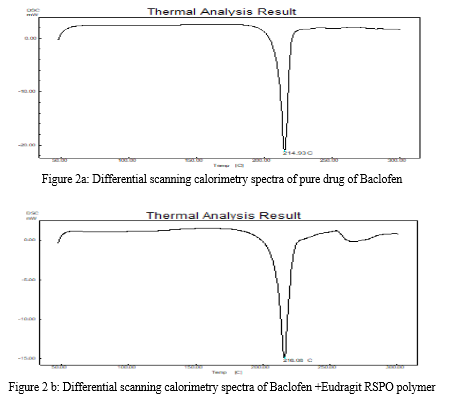
DSC study of baclofen, Eudragit RSPO and drug-loaded microballoons were carried out to study the stability of the drug during the formulation storage and Any abrupt or drastic change in the thermal behaviour of either the drug or polymers is tracked in the DSC thermograms which are portrayed in Figure 2. DSC curve of baclofen showed a sharp endothermic peak at 142.98oC, corresponding to its melting point. The polymer revealed an endothermic peak at 65.37oC indicating melting temperature of the polymer, whereas drug-loaded microballoons did not show any characteristic peak at 142.98oC suggesting that of baclofen existed as a molecular dispersion in the polymer matrix.
The microballoons revealed angle of repose values in the range of 30.80to 32.50 with Carr’s index value ranging from 7.79 to 11.13 indicating good flow properties. Further, the microballoons revealed Hausner’s ratio values in the range of 1.084 to 1.125 indicating good flow characteristics and not necessitating the incorporation of a glidant. The Micromeritic properties of the microballoons are enlisted in Table 2 and Table 3
Table.2: Micromeritic properties of Baclofen loaded Microballoons
|
Formulation code |
Particle size (µm)*
|
Bulk density (gm/ml) * |
Tapped density (gm/ml) * |
Hausner’s ratio* |
Carr’s Index* |
|
MB1 |
52.37±0.021 |
0439±0.008
|
0.494±0.016 |
1.125±0.238 |
11.1±0.235 |
|
MB2 |
61.62±0.043 |
0.492±0.005
|
0.539±0.007 |
1.095±0.267 |
8.71±0.231 |
|
MB3 |
63.50±0.056 |
0.532±0.008
|
0.5777±0.014 |
1.084±0.531 |
7.79±0.176 |
|
MB4 |
67.50±0.153 |
0.539±0.004 |
0.587±0.002 |
1.089±0.321 |
8.17±0.432 |
*Values are average of six readings ± standard deviation.
As the drug to polymer ratio was increased, the yield of the product also increased. The low percentage yield in some formulations may be due to microballoons lost during the washing process. The percentage yield was found to be in the range of 82 - 89%.
The % Drug entrapment efficiency of baclofen ranged from 80.65% to 97.62%. The drug entrapment efficiency of the prepared microspheres increased progressively with increase in proportion of the polymer. Increase in the polymer concentration increases the viscosity of the dispersed phase. The higher viscosity of the polymer solution at the highest polymer concentration would be expected to decrease the diffusion of the drug into the external phase which would result in higher entrapment efficiency. The percentage yield and % drug entrapment efficiency are displayed in Table 3.
Table.3: Physicochemical Characterization of Baclofen loaded Microballoons
|
Formulation code |
% yield* |
Entrapment Efficiency (%)* |
Angle of repose(degrees) *
|
Buoyancy (%)*
|
|
MB1 |
86.0±0.01 |
80.60±0.02 |
32.50±0.32 |
82.0±0.56 |
|
MB2 |
81.0±0.04 |
82.02±0.12 |
31.30±0.42 |
84.2±0.32 |
|
MB3 |
89.0±0.03 |
97.15±0.15 |
31.20±0.23 |
86.2±0.15 |
|
MB4 |
85.0±0.06 |
95.19±0.34 |
30.80±0.32 |
91.8±0.23 |
*Values are average of three readings ± standard deviation.
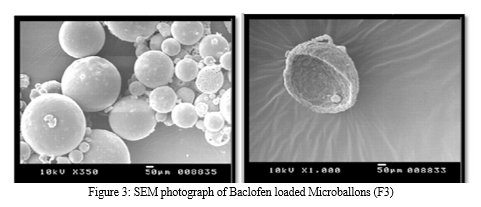
Morphology of the microspheres was investigated by Scanning electron microscopy. The photographs of the optimized formulations taken by scanning electron microscope are shown in the Figure.3. Surface topography of the spherical microballoons revealed a porous texture, thereby suggesting the possible drug release mechanism to occur by diffusion. The meanparticle size increased with increasing polymer concentration which is due to a significant increase in the viscosity, thus leading to an increased emulsion droplet size and finally a higher microballoon size. Baclofen microballoons had a mean particle size in the range of 52.37µm to 67.5µm.
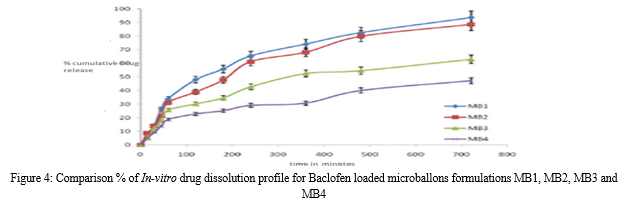
The In-vitro release of baclofen from the prepared microballoons exhibited a biphasic mechanism. The release of baclofen from the microballoons was characterized by an initial phase of burst effect (higher release), which was due to the presence of drug particles on the surface of the microballoons followed by a second phase of moderate release. The initial burst effect may be attributed as a desired effect to ensure initial therapeutic plasma concentration of the drug. The biphasic pattern of release is a characteristic feature of matrix diffusion kinetics. The initial burst effect was considerably reduced with increase in polymer concentration. The fact that increase in the polymer concentration resulted in better incorporation efficiency could be the reason for the observed decrease in burst effect, since the amount of surface associated drug decreases with an increase in entrapment efficiency.
As the polymer to drug ratio was increased, the extent of drug release decreased. A significant decrease in the rate and extent of drug release is attributed to the increase in density of polymer matrix that results in increased diffusion path length which the drug molecules have to traverse. The release of the drug has been controlled by swelling control release mechanism. Additionally, the larger particle size at higher polymer concentration also restricted the total surface area resulting in slower release.
With the increase in polymer to drug ratio, the formulations MB1and MB2 exhibited a % cumulative drug release of 93.29 % and 86.34% respectively at the end of 12 hrs.MB3 and MB4 exhibited a % cumulative drug release of 59.32% and 43.61% respectively at the end of 12 hrs.The drug release profiles are displayed in Figure.4.
For understanding the mechanism of drug release and release rate kinetics of the drug from dosage form, the obtained in vitro data were fitted to various mathematical models such as zero order, First order, Higuchi matrix, Korsmeyer-Peppas model and Hixson model using software (PCP-Disso resultant kinetic values are compiled in Table 4. Higuchi plot for different microballoon formulations is projected. The n values have been found to be in the range of 0.5133 – 0.5341, which is indicative of non-Fickian diffusion mechanism. The obtained Rvalues are closer in case of Higuchi model. Therefore, the drug release occurs by matrix diffusion controlled mechanism.
Table 4: Model fitting for drug release rates
|
Formulation code |
Zero order R2* |
First order R2* |
Higuchi R2* |
Korsmeyer PeppasR2* |
n value* |
|
MB1 |
0.8368±0.231 |
0.9718±0.126 |
0.9790±0.325 |
0.9562±0.789 |
0.5213±0.321 |
|
MB2 |
0.8717±0.342 |
0.9667±0.431 |
0.9877±0.321 |
0.9812±0.762 |
0.5277±0.632
|
|
MB3 |
0.8437±0.453 |
0.9356±0.453 |
0.9769±0.642 |
0.9256±0.832 |
0.5133±0.452
|
|
MB4 |
0.8723±0.452 |
0.9602±0.389 |
0.9768±0.452 |
0.9780±0.632 |
0.5341±0.329 |
*Values are average of six readings ± standard deviation.
IV. ACKNOWLEDGEMENT
Sincere thanks to our Principal C.L.BaidMetha College of Pharmacy, Chennai for this unstinted support and constant encouragement and guidance. Sincere thanks to faculties of C.L.Baid Metha College of Pharmacy, Chennai for providing the facilities for carry out the work.
V. CONFLICT OF INTEREST
The author declares that there is no conflict of interests regarding the publication of this paper.
Conclusion
In this investigation, we were able to successfully formulate gastroretentive hollow microballoons of baclofen. The procedure was simple, reproducible yielding hollow microballoons with a porous surface texture. The drug release from the formulation occurred by non-fickian and higuchi matrix diffusion controlled mechanism staining the drug release for a period of up to 12hrs based on the results of evaluation tests it could be concluded that MB3 and MB4 were the best formulations for oral sustained delivery of baclofen. The microballoons filled in a capsule need to b administered with a glass of water to provide suitable floatation. Thus the objective of designing a floating drug delivery system of baclofenhas been achieved with success.
References
[1] Gupta S, Mali RR, Goel V. Novel study in fast dissolving drug delivery system: A review. Indian J Pharm Biol Res. 2015; 3(1):93-107. [2] Nayak AK, Maji R, Das B.Gastroretentive drug delivery systems: A review. Asian J Pharm Clin Res. 2010;3(1):1-10. [3] Kiss D, Zelkó R. Gastroretentive dosage forms. Acta Pharm Hung 2005;75(3):169-76. [4] Hou SY, Cowles VE, Berner B. Gastric retentive dosage forms: A review. Crit Rev Ther Drug Carrier Syst. 2003;20(6):459-97. [5] Kawashima Y, Niwa T, Takeuchi H, Hino T, Itoh Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J Pharm Sci. 1992;81(2):135-40. [6] Pujara ND, Patel NV, Thacker AP, Raval BK, Doshi SM, Parmar RB. Floating microspheres: A novel approach for gastro retention. World J Pharm Pharm Sci. 2012;1(3):872-95 [7] Dasari N, Enjamuri A and Sudhakar M. Formulation and evaluation of baclofen floating tablets. Asian Journal Research Pharm. Science. 2016; 6(4): 255-60. [8] Gande S and Rao YM. Sustained-release effervescent floating matrix tablets of baclofen: Development, optimization and in vitro-in vivo evaluation in healthy human volunteers. DARU 2011; 19(3): 202-09. [9] Basavaraj BV, Deveswaran R, Bharath S, Abraham S, Furtado S, Madhavan V-Hollow microspheres of diclofenac sodium. Agastroretentive controlled delivery system. Pak. J. Pharm. Sci. 2008; 21(4): 451-54. [10] Trivedi P, Verma AM, Garud N. Preparation and characterization of aceclofenac microspheres. Asian J Pharm 2008;2(2):110-5. [11] Martin A, Butamante P, Chun AH. Physical Pharmacy. 4th ed. Philadelphia, PA: Lea & Febiger,1993. pp. 431. [12] Manavalen R, Ramasamy C. Physical Pharmaceutics. 2nd ed. Tamil Nadu: Vignesh Publisher, 2001. pp. 456.Sinha VR, Agrawal MK, Kumria R. Influence of formulation and excipient variables on the pellet properties prepared by extrusion spheronization. Curr Drug Deliv. 2005;2(1):1-8 [13] Gattani YS, Bhagwat DA, Maske AP. Formulation and evaluation of intragastric floating drug delivery system of diltiazem hydrochloride. Asian J Pharm 2008;2(4):228-31. [14] USP. The United States Pharmacopoeia XXIV. Rockville, MD: United States Pharmacopoeial Convention; 2000. pp. 1941. [15] Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. Physicochemical properties to determine the buoyancy of hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method. Eur J Pharm Biopharm. 2003;55(3):297-304. [16] Kawashima, Y.; Niwa, T.; Takeuchi, H.; Hino, T.; Itoh, Y. Preparationof multiple unit hollow microspheres (microballoons) withacrylic resin containing tranilast and their drug release characteristics (in vitro) and floating behavior (in vivo). J. Control. Release.1991;16,
Copyright
Copyright © 2022 J. Gomathi, T. Muthukumaran, Kavitha. C, Priyanka Sinha, Sabbathyan Balla. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET46820
Publish Date : 2022-09-19
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

