Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Optimizing the Gas Carburization Process: Strategies for Reducing Total Cycle Time
Authors: Husam Elkaseh
DOI Link: https://doi.org/10.22214/ijraset.2023.48642
Certificate: View Certificate
Abstract
This case study presents the results of an experiment designed to reduce the total cycle time of the gas-Carburizing process for AISI 8620 steel. By optimizing process parameters such as carburizing temperature, soaking time, and carbon potential, the effective case depth was significantly improved. Previous research has indicated that optimizing the duration of the boost/diffusion stages and carbon potential in the carburizing process can lead to a 25% reduction in total process time [1].Our study showed that it is possible to achieve a reduction in total cycle time of up to 21%, leading to cost savings for heat treatment companies while also promoting a safer environmental outcome
Introduction
I. INTRODUCTION
Carbon profiles have been predicted through various methods including calculations, and computer simulations such as CarbCALCII. However, these predictions tools do not always agree with experimental results. The importance of achieving the desired carbon profile lies in the need for the correct carbon content at a specific depth to ensure that final products meet all required mechanical and metallurgical specifications.
Accurate prediction of the carburization profile requires a thorough understanding of the diffusion coefficient of carbon in the austenite phase of the steel being used as well as the mechanisms of carbon diffusion. This will be discussed in further detail in this paper, with additional information available in references [3] and [4].Top of Form
A. The Carburizing Process
It is important to understand the chemical reactions that leads to produce the carbon along with the diffusion theory.
Carburizing gases in this experiment are produced in two steps. First, natural gas is mixed with air in endothermic gas generator. The mixture then passes throw catalytic Ni at 1850 F° and burns incompletely. The outcome gases are cooled and dehydrated to become a mixed gas (endothermic gas), with a ratio of N2:CO:H2 40:20:30, Dewpoint +40, and air to gas ratio 2.5:1
Endothermic carburizing atmosphere consists of CO, H2, and N2 together with CO2, H2O and CH4 [2]. These gases provide the sources of carbon atom by thermal chemical reaction.
In order to fully understand the carburizing process, it is important to be familiar with the chemical reactions that produce carbon and the underlying diffusion theory.
The carburizing gases used in this experiment were generated through a two-step process. First, natural gas was mixed with air in an endothermic gas generator, and the mixture was passed through a catalytic Ni at 1850°F, where it underwent incomplete combustion. The resulting gases were cooled and dehydrated to form a mixed gas (endothermic gas) with a composition of N2:CO:H2, a dewpoint of +40, and an air-to-gas ratio of 2.5:1. The endothermic carburizing atmosphere consists of CO, H2, and N2, as well as CO2, H2O, and CH4 [2], which provide the sources of carbon atoms through thermal chemical reactions.
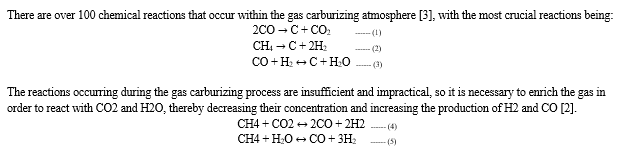
There are over 100 chemical reactions that occur within the gas carburizing atmosphere [3], with the most crucial reactions being:
B. Carbon Transfer
The mechanism of carbon transfer during gas carburizing is a complex process that comprises three distinct stages. The first step involves the absorption of carbon molecules from the gas onto the surface of the steel, driven by a higher concentration in the atmosphere compared to the surface area. The second step involves the dissociation of the gas at the surface. The third step involves the absorption and diffusion of carbon atoms into the bulk of the steel. A flux balance is employed to calculate the carbon flux at the surface and carbon diffusion to measure the penetration of carbon into the steel (see Figure 1).
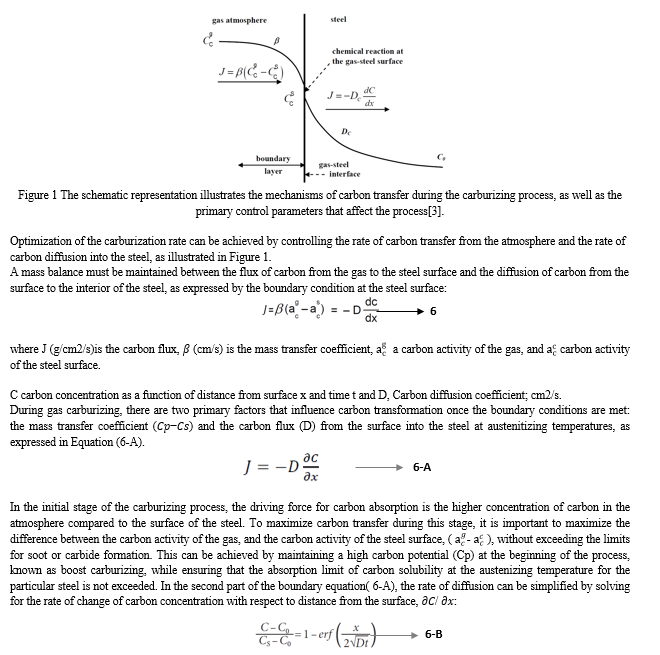

II. EXPERIMENT AND MATERIAL
A. Boost & Diffusion
The gas carburizing process is known for its lengthy duration, leading to the development of the boost/diffusion method for minimizing the total time required to achieve a specific case depth. In an effort to reduce the duration of the carburization process, various proposals have been made, including the use of software, computer simulations, and complex equations to accurately predict the final carbon profile of gas carburizing. In this analysis, we will examine some prediction tools that are available on the market and compare their results to experimental data.
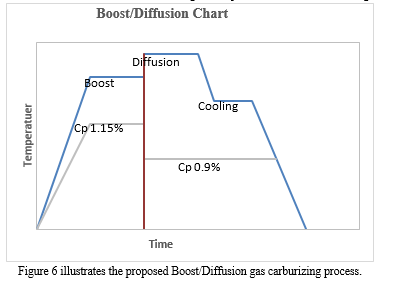
During the initial stage of gas carburizing, the carbon potential is increased to create a buildup of carbon on the part being treated. Most commercial gas carburizing furnaces are designed to operate at a maximum temperature of around 1700 °F to extend the lifespan of the equipment. For this reason, we will be using a gas carburizing furnace with a maximum temperature of 1700 °F, provided by Atlantic Heat Treat in Canada. The material used in this experiment is AISI 8620, as listed in Table 1. The carbon potential can be selected based on the maximum solubility of carbon from the Acm line in the carbon iron phase diagram at 1700F, which is approximately 1.21% C. During this experiment, we encountered a limitation with the Endo gas generator used, which produced a lower amount of H2O/CO2, resulting in a longer time to reach 1.21% C. As a result, the Cp was set to 1.15% C in the boost stage and 0.9% C in the diffusion stage using a programmable controller.
|
Table 1 Chemical composition of 8620 AISI [3]. |
|
|
Cr |
Fe |
Mn |
Mo |
Ni |
P |
S |
Si |
Cr |
|
8620 |
0.214 |
0.560 |
Bal. |
0.805 |
0.1535 |
0.4525 |
0.0065 |
0.014 |
0.2355 |
During the diffusion stage, the rate of carbon transfer becomes limited by the carbon diffusion in austenite. As shown in equation (7), it is generally more advantageous to set the diffusion segment at the highest possible temperature. While software may recommend lowering the temperature during diffusion, this does not typically result in a shorter cycle time or deeper case depth, as will be demonstrated later.
B. Experiment and Procedure
The total cycle time was calculated mathematically using equation (7) for 0.04, 0.06, and 0.08 inch depth, as shown in Table 2. This calculation was then compared to the total time measured experimentally using the same values. In the experimental portion of the study, the total cycle time was calculated and then divided by 2, based on a boost/diffusion ratio of 0.5 (r) at 1700F [2], as shown in Figure 4. The total run cycle scheme is illustrated in Figure 4, comprising the boost, diffusion, cool, and quench stages. The Cp was set to 1.15% C in the Boost segment at a temperature of 1700 F, and then decreased to 0.9% C in the diffusion segment at the same temperature.
All specimens of 8620 material were cut into cubic shapes measuring 1cm x 1cm x 2cm, cooled down to 1575F for 20 minutes before quenching. This time was not included in the total cycle time as it was set at 20 minutes for all tests and can vary in actual production depending on the size and load of the parts being heat treated. Finally, after quenching, the effective case depth was measured according to ASTM B934, as shown in Figure 3
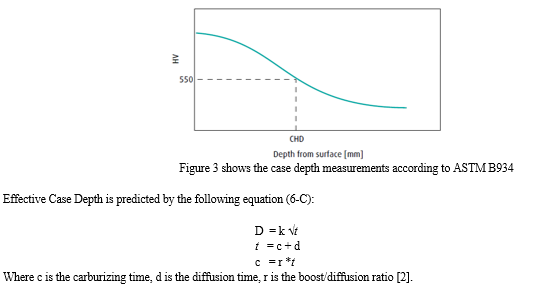
Table 2 Total run time vs ECD.
|
Effective Case Depth-ECD |
Temp |
Calculated total-time, hr |
|
0.04 |
1700 |
4 |
|
0.06 |
1700 |
6 |
|
0.08 |
1700 |
16 |
|
0.1 |
1700 |
25 |
III. PERFORMANCE TESTING AND OBSERVATION
A. Testing
Four trials were conducted to assess the impact of the boost/diffusion method on the ECD and total cycle time, with the goal of minimizing the total run time without compromising the effective case depth. All tests were performed using the total calculated cycle time from Table 2, which was divided into boost and diffusion segments with Cp values of 1.15% and 0.9%, respectively, as shown in Figure 4. The ECD was measured and the time was adjusted until the desired case depth was achieved or it was determined that no further time could be deducted.
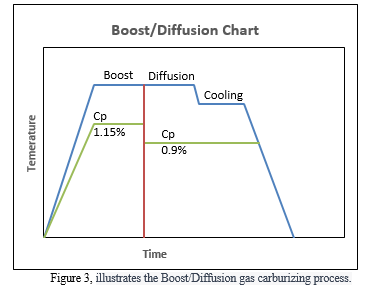
Testing
Test 01 - (0.04”)
In test #1, 240 minutes total run time was applied see table 2. The total run time was then divided by 2 ( 240 minutes/ 2= 120 minutes for each step). Finally, the ECD was measured and found to be at 0.05” after 240 minutes of processing time.
In Test #1, a total run time of 240 minutes was applied, as shown in Table 2. The total run time was then divided in half, resulting in 120 minutes for each step. Upon measurement, the ECD was found to be 0.05 inches after 240 minutes of processing time
|
ECD-inch |
Boost, Time, mins |
Carbon potential |
Carburizing, Time, mins |
Carbon potential |
Cool to 1575F,min |
Measured ECD, inch |
Reduction % |
|
|
0.04 |
#1 |
120 |
1.15 |
120 |
0.9 |
30 |
0.050 |
0% |
|
#2 |
100 |
1.15 |
100 |
0.9 |
30 |
0.045 |
-17% |
|
|
#3 |
90 |
1.15 |
90 |
0.9 |
30 |
0.041 |
-25% |
|
Test 02 - (0.06”)
|
ECD-inch |
Boost, Time, mins |
Carbon potential |
Carburizing, Time, mins |
Carbon potential |
Measured ECD, inch |
Reduction % |
|
|
0.06 |
#1 |
270 |
1.15 |
270 |
0.9 |
0.069 |
0% |
|
#2 |
240 |
1.15 |
240 |
0.9 |
0.066 |
-11% |
|
|
#3 |
210 |
1.15 |
210 |
0.9 |
0.060 |
-22% |
|
Test 03 - (0.08”)
|
ECD-inch |
Boost, Time, mins |
Carbon potential |
Carburizing, Time, mins |
Carbon potential |
Measured ECD, inch |
Reduction % |
|
|
0.08 |
#1 |
480 |
1.15 |
480 |
0.9 |
0.085 |
0% |
|
#2 |
420 |
1.15 |
420 |
0.9 |
0.083 |
-12.5% |
|
|
#3 |
380 |
1.15 |
380 |
0.9 |
0.08 |
-20.8% |
|
Test 04 - (0.1”)
|
ECD-inch |
Boost, Time, mins |
Carbon potential |
Carburizing, Time, mins |
Carbon potential |
Measured ECD, inch |
Reduction % |
|
|
0.100 |
#1 |
750 |
1.15 |
750 |
0.9 |
0.108 |
0% |
|
#2 |
x |
1.15 |
x |
0.9 |
x |
X |
|
|
#3 |
610 |
1.15 |
610 |
0.9 |
0.104 |
18.6% |
|
|
#4 |
600 |
1.15 |
600 |
0.9 |
0.1 |
20% |
|
IV. RESULTS AND DISCUSSION
It is evident that the carbon boost has an impact on the effective case depth. In Test #1, the total time was reduced by 25% after increasing the Cp to 1.15%, resulting in a savings of one hour of time and energy. In Test #4, the time was decreased from 750 to 600 minutes, resulting in an average case depth of 0.1 inches and a saving of five hours. Due to furnace availability, it was not possible to conduct further testing at long cycle times. Therefore, the next attempt aimed to achieve the desired ECD of 0.1 inches in as few trials as possible. The total time was set to 1220 minutes, a reduction of 18.6% based on the results of the previous three tests, which showed reductions of 25%, 22%, and 20.8%, as shown in Figure 5.\
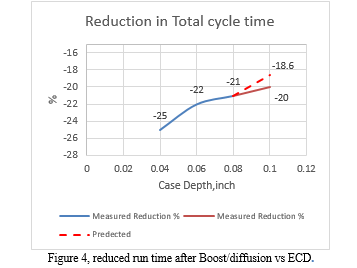
The effective case depth was predicted to be 0.1 inches after a time reduction of 18.6%. However, the measured ECD was 0.104 inches, which is 0.004 inches higher. This indicates that the relationship between ECD and time reduction is not linear, as shown in Figure 5. In fact, the curve appears to plateau after the total run time reaches 12 hours. This suggests that deeper cases can only be achieved with longer cycles when the diffusion stage temperature is fixed. This claim is supported by the carbon diffusivity equation, which demonstrates that diffusivity at this stage of carburizing is primarily a function of temperature, as shown in Equation 9 and Figure 2. The only way to reduce the diffusion time is to increase the temperature, which is not an option for many commercial gas carburizing furnaces. Therefore, it may be worth considering alternative boost/diffusion parameters for longer cycles, such as longer boost times and shorter diffusion times at higher temperatures, as illustrated in Figure 6.
A. Alloying Elements.
It was previously mentioned that the alloying elements of the steel play a significant role in predicting the ECD. For low carbon steel, the influence may be minimal, but for medium and high alloy steels, the effect of alloying elements can be significant and should be taken into account.
This was demonstrated by case hardening 4140 steel to a depth of 0.04 inches and comparing it to 0.04 inches of case hardened 8620 steel. The 4140 steel was heat treated using the same conditions as Test 01 (0.04 inches) with a 25% time reduction. The result showed that the ECD of case hardened 4140 steel was 0.03 inches, which is 0.01 inches less than the 8620 steel treated under the same conditions. While this difference may seem acceptable due to the difficulty of maintaining a tolerance of less than 0.01 inches in depth, it still demonstrates that alloying elements can significantly impact the ECD.
It should also be noted that during experimentation with different specimens of the same type of steel grade, the ECD results can vary due to differences in the origin of the steel. Steel produced in one country may exhibit different reactions to heat treatment compared to the same steel grade produced by another manufacturer. Heat treaters must consider these factors as a result of variations in steel quality from different manufacturers.
???????B. Cab Software
It is clear that computer modeling and simulation tools can provide valuable insights into the gas carburizing process, but it is important to note that they are not always accurate and should be compared to experimental results to ensure their validity. One such tool, CarTool, was developed by the Center of Heat Treatment Excellence and utilizes boundary condition equations based on carbon diffusivity to predict the ECD and necessary process for various steel types. However, when applied to the low pressure carburization of 9310 AISI steel, the simulated carbon profile differed significantly from the experimental results, as depicted in Figure 7.
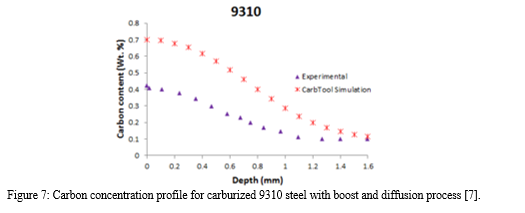
In the aforementioned example, it is demonstrated that computer model predictions may not always align with experimental results, as was the case with 9310 steel. This discrepancy may be due to the formation of carbides and soot on the surface of the part, which hinders the flow of carbon, or due to the fact that the quality of the steel is not a fixed parameter in the simulation. Therefore, it is important to verify the effectiveness of a chosen gas carburizing procedure through experimental testing.
While computer modeling can be helpful in reducing the number of trials needed to achieve the desired carbon profile, it is ultimately necessary to conduct physical testing to confirm the effectiveness of the chosen procedure.
??????????????C. Recommendations
It is recommended that when case hardening any steel, the total time required to achieve the desired ECD is calculated upfront to ensure that the process is initiated with a target range in mind. One method for achieving this is to utilize the Harris equation [6] and corresponding carburizing constant k [2], as this approach is both simple and cost-effective while also providing accurate ECD predictions.
Experimenting with boost/diffusion setups for a particular steel can also be vital in minimizing total run time.
This research also suggests that it may be worth further examining the possibility of lowering boost temperatures, as the high levels of carbon in the atmosphere during this stage significantly impact the carbon profile. The effects of multiple short boost/diffusion cycles and the impact of higher temperature and lower Cp during diffusion, following the buildup of high levels of carbon in the steel bulk, should also be studied further.
Conclusion
Carburizing is a non-steady-state diffusion process, making it difficult for scientists and heat treaters to accurately predict the effective case depth. Any proposed strategies for limiting the gas carburizing process will face two main challenges. Firstly, raising the carbon potential too high can lead to the formation of soot and carbides at the outer edge of the part. Secondly, increasing the temperature to improve carbon diffusivity is generally impractical due to cost and furnace lifespan considerations It has been demonstrated that deeper case depths in gas carburizing may only be achieved through the use of longer process cycles. Factors such as carbon potential and temperature can be manipulated to some extent to minimize the duration of the process, but ultimately the limitations of soot and carbide formation as well as the practical considerations of cost and furnace lifespan make it necessary to accept longer process times in order to achieve deeper cases.
References
[1] Dosset, Jon L. and Howard E. Boyer. “Practical Heat Treating: Second Edition.” (2006). [2] ASM Handbook, Volume 04 - \"Heat Treating\" (1991) [3] Karabelchtchikova, Olga. “Fundamentals of Mass Transfer in Gas Carburizing.” (2007). [4] G. Parrish and G. S. Harper. \"Production Gas Carburising: First Edition\" (1985) [5] Li, Ma. “Practical Approach to Determining Effective Case Depth of Gas Carburizing.” (2016). [6] Herring, Daniel H. and Jeremy C. St. Pierre, “Vacuum Carburizing of P/M Steels.” Industrial Heating, (1987) [7] Zhang, Lei. “Modeling and Verification of Simulation tools for Carburizing and Carbonitriding.” (2017).
Copyright
Copyright © 2023 Husam Elkaseh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET48642
Publish Date : 2023-01-13
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

