Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Synthesis, UV-Vis Spectroscopic and Physico-chemical Characterization of Oxovanadyl Complexes of Lincomycin and Neomycin
Authors: Olufunso O. Abosede, Charles G. Ikimi
DOI Link: https://doi.org/10.22214/ijraset.2022.39801
Certificate: View Certificate
Abstract
In the recent past, the pharmaceutical modification of drug molecules by complexation with biologically relevant metals to improve their properties such as stability, dissolution rate, absorption and bioavailability has been extensively studied. In order to achieve better and enhanced medicinal activity, vanadyl complexes of the widely used lincomycin (Lin-van) and neomycin (Neo-van) have been synthesized and their physico-chemical properties examined. The UV-Vis absorption properties of these complexes were determined and their antimicrobial activities were tested against some pathogenic organisms viz: Proteus vulgaris, Klebsiella pneumonae, Escherichia coli and Staphylococcus aureus. In all cases, Neo-van showed better antimicrobial activity than Lin-van while both complexes showed better activity than the antibiotic lincomycin and the previously reported Cu-Lin.
Introduction
I. INTRODUCTION
Diverse metal ions are present in biological systems and have been found to play important roles in different enzymatic and physiological processes. Essentials elements such as vanadium and their compounds are widely found in nature and play crucial roles in biological systems including in proteins, enzymes and co-enzymes. For example, vanadyl salts have been employed as anti-diabetes for more than a century and vanadium (IV) and vanadium (V) complexes have explored for their insulin-mimetic properties [1]. Significant number of publications has appeared on the biological role different oxidation states of vanadium in medicine [2]. VO2+ is referred to as the most stable existing diatomic ion which forms diverse stable cationic, anionic and neutral complexes when coordinated to a large variety of ligands particularly with most electronegative donor groups [3]. Major studies on vanadium have focused on the development of vanadium(IV) or -(V) complexes with organic ligands as delivery agents that improve gastrointestinal absorption and reduce the toxicity of vanadate [4], [5].
The role of metal complexes in relation to biological activity has been recently pursued [2], [6]. The features of some drugs enable them to play vital role as bio-ligands in the biological systems. Similarly, the activity of the bio-metals is acquired through the formation of complexes with different bio-ligands and the thermodynamic and kinetic properties of the complexes determine the mode of biological action. Sometimes, the lipophilicity of drugs which imparts permeability is enhanced through the formation of complexes in vivo and drug activity is substantially augmented due to much more effective penetration of the drug into the site of action. Many drugs substances, such as antimicrobials and anticancer agents exhibit their drug action through coordination with available bio-metals in the biological systems. Interaction of various metal ions with organic-based drugs may improve or diminish the antimicrobial activity of the drugs. In many cases, the pharmacological activity of antibiotics, after complexation with metals, is increased when compared to that of the free ligands [6]. Many of the commonly used antibiotics such as penicillins, aminoglycosides, tetracyclines are chelating agents and their biological activity is enhanced in the presence of bio-metals [7], [8].
Transition metal complexes of organic-based ligands possess some advantages over metal-free drugs. These advantages are due to their ability to coordinate ligands in a three dimensional conformation, thus allowing functionalization of groups that can be harnessed for specific molecular targets. The partially filled d orbitals in transition metals convey additional electronic properties while the oxidation state of a metal is also an important factor in designing metal-based drugs. The metal ion and its oxidation state allow the participation of metal-based compounds in biological redox reactions and play an important role in determining the dosage and bioavailability of the administered agent. In addition, the ability to undergo ligand exchanged reactions confers on the metals the ability to interact and coordinate to biological molecules as revealed by the widely used drug cisplatin.
Organic-based drugs are proven to be good chelating agents of metals and are avid ligands in the formation of metallotherapeutics. Metalloatherapeutics can interact with several different kinds of biomolecules, including DNA, RNA, proteins, receptors and lipids, making them very unique. Physical, chemical and biological changes such as stability, binding behavior, solubility, biovailability, absorption in organic-based drugs have been reported after their interaction with metals or biological components as metallotherapeutics [4]. Metals may produce synergistic, antagonistic and toxic effects on complexation with organic-based drugs. Hence the complete biological study along with the stability and binding behavior is required for the synthesis of various metal-containing drugs [8]). Remarkable examples among vanadium-containing therapeutics are vanadium-based bis(maltolato), picolinato and thiazolidinedione compounds for diabetes therapy [4].
Lincomycin and neomycin (Figure 1) are well known broad spectrum antibiotics belonging to the class of lincosamides and aminoglycosides antibiotics respectively. Neomycin is a constituent of many topical medications such as creams, ointments, and eye drops. Neomycin was first discovered in 1949 in the laboratory of Selman Waksman when it was isolated from Actinomycetes together with streptomycin ( http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/selmanwaksman.html ). As an aminoglycoside antibiotic, neomycin contains two or more aminosugars connected by glycosidic bonds and is active against gram negative and gram positive bacterial. However, widespread resistance to theses antibiotics has been reported [9], [10] and this has necessitated possible improvement of these antibiotics. In view of this, oxoanadium (II) complexes were formed with lincomycin (Lin-van) and neomycin (Neo-van). The visible spectra and physico-chemical properties of these complexes were determined and their antimicrobial activities were tested against some pathogenic organisms.
II. EXPERIMENTAL
A. Materials, Instrumentation and Methods
- Materials and Instrumentation: All reagents were of analytical grade and were used as supplied from commercial sources. Neomycin sulphate and vandyl sulphate hydrate were from VWR England. Lincomycin hydrochloride was a donation from Drugfield pharmaceuticals, Sango-Otta, Nigeria. Solutions were prepared using deionized water. The solutions of drugs and metal salts were prepared in deionized water. UV-Vis spectra were recorded in 1.0 cm path length quartz cell on JASCO V-730 UV-Vis spectrophotometer.
- Synthesis of Neomycin-vanadyl complex (Neo-van): 1.0 mmole (0.908 g) of neomycin sulphate was dissolved in 3 ml deionized water forming a yellow solution. 1.0 mmole (0.181 g) of vanadyl sulphate hydrate was also dissolved in 3 ml of deionized water, a blue colour was observed. The vanadyl sulphate solution was added to the neomycin solution. On addition of vanadyl sulphate solution to neomycin solution a light green colour was observed. The reaction was stirred at room temperature for 1 hour, 20 minutes after which the colour was observed to change from light green to dark green colour. Microcrystalline products formed after slow evaporation of the mother liquor after 2 months were filtered and stored in well-capped vial.
- Synthesis of Lincomycin-vanadyl complex (Lin-van): 1.0 mmole (0.443 g) of lincomycin hydrochloride was dissolved in 3 ml deionized of water. 1.0 mmole (0.181g) of vanadyl sulphate hydrate was also dissolved in 3 ml of deionized water forming a blue solution. The vanadyl sulphate solution was added to the neomycin solution. On addition of vanadyl sulphate solution to the neomycinsolution, a light green solution was formed. The reaction was stirred at room temperature for 1 hour, 20 minutes after which a dark green solution was formed. Microcrystalline products formed after slow evaporation of the mother liquor after 2 months were filtered and stored in well-capped vial.
- Antimicrobial Analysis: The complexes were tested for their antimicrobial activity by agar well diffusion method. In this method the pre-sterilized petri-dish containing 20 ml of nutrient agar was allowed to solidify. Then bacteria were introduced aseptically using striking plate method. A sterile cork borer was used to make wells of 8 mm in diameter in each of the plates. Suitable dilutions of the complexes were made with sterile water and carefully placed in each well by micro pipette. The plates were incubated at 37 °C for 24 hours. Antimicrobial activity was evaluated by measuring the inhibition zones. Inhibition zones were recorded as the diameter of no growth area.
B. Physico-chemical Analysis
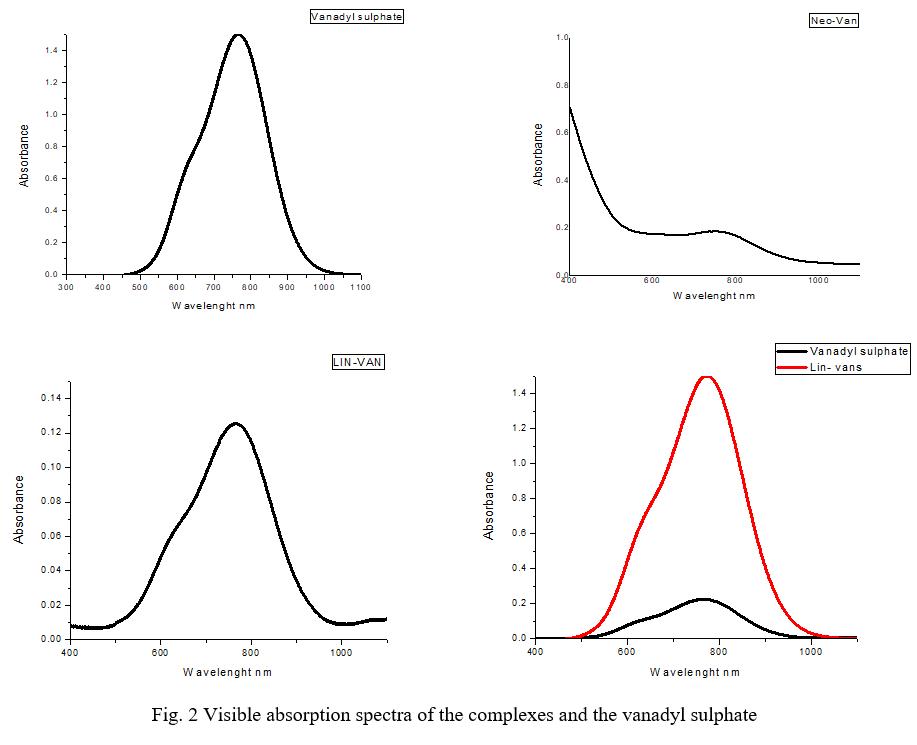
The pharmaceutical modification of drug molecules by complexation with biologically relevant metals to improve their properties such as stability, dissolution rate, absorption and bioavailability has been an increasingly growing area of research because of the first success of cisplatin, an inorganic anticancer drug. The complexes have been synthesized by the reaction of aqueous solutions of vanadyl sulphate and active pharmaceutical ingredients (Neomycin and Lincomycin) in an equimolar ratio. The reaction mixtures (vanadyl-neomycin, vanadyl-lincomycin) were stirred at room temperature. The resulting microcrystalline products obtained after evaporation of the mother liquor at ambient conditions were filtered and stored in a vial. The complexes have different colours from those of the ligands and metal salt. Oxoanadium complexes were successfully formed with lincomycin (Lin-van) and neomycin (Neo-van). As the complexes are expected to show d-d transitions in the visible region, the electronic spectra of the metals and complexes have been studied in the range of 400-1100 nm. Each of the complexes studied showed absorption in visible spectra. The visible spectra confirm the formation of metal-ligand complexes.
Table 1 shows the solubility of the complexes and antibiotics in different solvents while Table 2 shows the physico-chemical parameters of the complexes.
Table ISolubility of the Complexes and Antibiotics
|
Compound |
Solubility in different solvents |
|||
|
Water |
Acetone |
Ethanol |
Methanol |
|
|
Lin – van |
Soluble |
Insoluble |
Soluble |
Soluble |
|
Neo- van |
Soluble |
Insoluble |
Slightly soluble |
Slightly soluble |
|
Lincomycin |
Soluble |
Partially soluble |
Insoluble |
Partially soluble |
|
Neomycin |
Soluble |
Insoluble |
Insoluble |
Insoluble |
Table II Physical Parameters of Ligands And Their Metal Complexes
|
Sample Ligands/metal complexes |
Colour |
Appearance |
|
Lin – van |
Green |
Crystal |
|
Neo – cu |
Blue |
Crystal |
|
Neomycin |
Yellow |
Powder |
|
Lincomycin |
Light yellow |
Crystalline powder |
Neo- van complex is soluble in water insoluble in acetone and partially soluble in methanol and ethanol while Lin-van complex is soluble in water, ethanol and methanol. This shows that Lin-van has improved solubilty especially in ethanol and methanol. The visible absorption parameters of the vanadyl complexes and vanadyl sulphate are presented in Table 3.
C. Visible Absorption Spectra
Table IIIVisible Absorption Spectra Of The The Metal Complexes And Vanadyl Sulphate
|
Complexes |
Wavelength of maximum absorption (λmax, nm) |
Assignment |
|
Neo –van |
750 |
d-d electronic transition ( 2B2g→E) |
|
Lin – van |
780 |
d-d electronic transition ( 2B2g→E) |
|
Vanadyl sulphate |
780 |
d-d electronic transition ( 2B2g→E) |
The peaks at the range 750-780 nm were assigned to the d-d electronic transitions of the type 2B2g→ E (dxy → dxz) of the VO(II) complexes. The wavelength of absorption of 750 and 780 show square pyramidal geometry around vanadium consisting of coordination of aqua ligands in addition to coordination of the ligands to vanadium to complete the square pyramid. As in copper complexes of lincomycin and neomycin, the oxovandium center is coordinated via the O-donor group of the antibiotic ligands. Figure 2 presents the visible absorption spectra of the complexes. Their spectral features showed their bonding and electronic transitions. The complexes are 1: 1 as in Cu(II) complexes [11], [12].
D. Antimicrobial Analysis
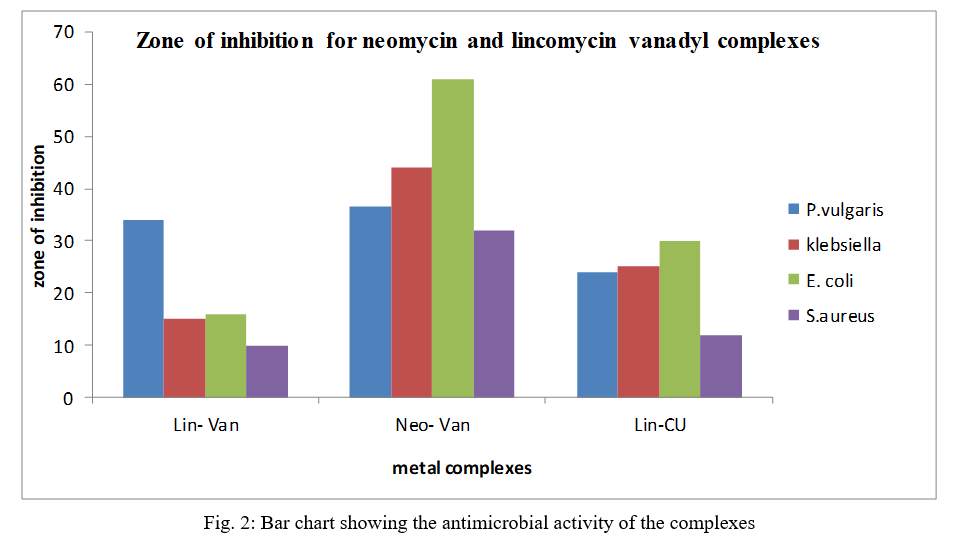
Antibiotics are substances which, even at low concentrations, inhibit the growth and reproduction of bacteria. The treatment of infectious diseases would be inconceivable today without effective antibiotics. Most of the existing antibiotics were originally derived from microorganisms and from plants. The newly synthesized metal complexes were screened for antibacterial activity against gram-positive bacteria (staphylococcus aureus) and gram-negative bacteria (Escherichia coli, proteus vulgaris and klebsiella), From Table 4, all metal complexes were found to inhibit all tested bacteria at different rates. From the test results, it was observed that the complexes exhibited excellent activity against almost all the strains of bacteria under study. Lin-Van showed zone of inhibition of 10 mm against Staphlococcus aureus, 16 mm against Escherichia coli, 15 mm against Klebsiella pneumonae, and 34 mm for Proteus vulgaris. Neo-Van showed zone of inhibition of 32 mm against Staphlococcus aureus, 61 mm against Escherichia coli, 44 mm against Klebsiella pneumonae and 36 mm for Proteus vulgaris. Neo-Vanadium complex showed better activity against all the tested organisms—Escherichia coli, Staphlococcus aureus, Proteus vulgaris and Klebsiella pneumonae than Lin-Van and its analogoue Lin-Cu whereas Lin-Van showed better activity than Lin-Cu.
All complexes have higher inhibition than the free ligand lincomycin. This improved antimicrobial activity of metal complexes can be explained on the basis of Tweedy’s chelation theory. On chelation, the polarity of the metal ion will be reduced to a greater extent due to the overlap of the ligand orbital and partially sharing of the positive charge of the metal ion with heteroatom donor atoms of the ligand. Also, the delocalization of the pie electron over the whole newly formed chelate ring increases thereby enhancing the penetration of the complex (due to increased lipophilicity) into the lipid membrane of the organisms and blocks the metal binding sites in the enzymes of microorganism . In these ways, the complexes disturb the respiration process of the cell and thus block the synthesis of protein, which restrict further growth of the organism [15].
The antimicrobial potencies in terms of zone of inhibition of the metal complexes and lincomycin are compiled in Table 4 and compared to licomycin and previously reported Cu-Lin [12].
Table IV Zone of Inhibition for Metal Complexes of Neomycin and Lincomycin
|
Metal complexes and control |
Zone of inhibition diameter (mm) against different organisms |
||||
|
Proteus vulgaris |
Klebsiella pneumonae |
Escherichia coli |
Staphylococcus aureus |
Reference |
|
|
Lin-van |
34.0 |
15.0 |
16 |
10.0 |
This work |
|
Neo-van |
36.0 |
44.0 |
61 |
32.0 |
This work |
|
Lin- Cu |
24.0 |
25.0 |
30.0 |
12.0 |
Obaleye and Abosede, 2019b |
|
Lincomycin 500 mg (control) |
R |
R |
R |
R |
This work |
The antimicrobial activities of the complexes tested against some pathogenic organisms viz: Proteus vulgaris, Klebsiella pneumonae, Escherichia coli and Staphylococcus aureus showed that Neo-van has the highest antimicrobial potency in all cases followed by Lin-Van and the previously reported Cu-Lin. The increased activity of the complexes is attributed to complexation to vanadium. Lincomycin was observed to be resistant to the organisms at the tested concentration of 500 mg and the experimental conditions. Figure 3 shows the pictogram/bar chart of the activities of the metal complexes.
III. ACKNOWLEDGMENT
Olufunso O. Abosede appreciates International Foundation for Science (IFS), Stockholm, Sweden and the Organization for the Prohibition of Chemical Weapons (OPCW) for the donation of JASCO UV-Visible V730 spectrophotometer through IFS individual grant IFS 5780-1.
Conclusion
Vanadyl complexes of lincomycin and neomycin have been synthesized and characterized by UV-Visible spectroscopic technique and their physico-chemical parameters. Their spectral features showed their bonding pattern and electronic transitions and that the complexes are 1: 1. Antimicrobial evaluation of the metal complexes against gram positive and gram negative bacterial such as Proteus vulgaris, Klebsiella pneumonae, Escherichia coli, Staphylococcus aureus showed higher activity on the tested bacteria than metal-free antibiobic licomycin. The metal complexes also possess good solubility in different solvents especially aqueous solubility.
References
[1] D. C. Crans, “Antidiabetic, Chemical, and Physical Properties of Organic Vanadates as Presumed Transition-State Inhibitors for Phosphatases”, J. Org. Chem., vol. 80, pp. 11899–11915, 2015. https://doi.org/10.1021/acs.joc.5b02229 [2] A. Azam, M. A. Raza, and S H. Sumrra, “Therapeutic Application of Zinc and Vanadium Complexes against Diabetes Mellitus a Coronary Disease: A review” Open Chemistry, vol. 16, pp. 1153-1165, 2018. https://doi.org/10.1515/chem-2018-0118 [3] J. Krakowiak, D. Lundberg, and I. Persson, “A coordination chemistry study of hydrated and solvated cationic vanadium ions in oxidation states +III, +IV, and +V in solution and solid state.” Inorg. Chem., vol. 51(18), pp. 9598–9609, 2012. https://doi.org/10.1021/ic300202f [4] A. Levina, A. I. McLeod, A. Pulte, J. B. Aitken, and P. A. Lay, “Biotransformations of Antidiabetic Vanadium Prodrugs in Mammalian Cells and Cell Culture Media: A XANES Spectroscopic Study” Inorg. Chem., vol. 54, pp. 6707?6718, 2015. DOI: 10.1021/ic5028948 [5] E. V. Fedorova, A. V. Buryakina, A. V. Zakharov, D. A. Filimonov, A. A. Lagunin, V. V. Poroikov, “Design, Synthesis and Pharmacological Evaluation of Novel Vanadium-Containing Complexes as Antidiabetic Agents”, PLoS ONE vol. 9(7) pp. e100386, 2014. https://doi.org/10.1371/journal.pone.0100386 [6] J. A. Obaleye and O. O. Abosede, “Fe(III)-Doxycycline Complexes with Diimine Ligands: Syntheses, Characterization and Biological Properties”, Maced. J. Chem. Chem. Eng, vol.. 38, pp. 29–38, 2019. DOI:10.20450/mjcce.2019.1506 [7] O. O. Abosede, N. A. Vyas, S. Singh, A. S. Kumbhar A. Kate, A. A. Kumbhar, A. Khan, A. Erxleben, P. Smith, C. de Kock, F. Hoffmann and J. A. Obaleye, “Copper (II) Mixed Ligand Polypyridyl Complexes with Doxycycline- Structures and Biological Evaluation”, Dalton Trans., vol. 45, pp. 3003–3012, 2016. http://dx.DOI:10.1039/C5DT04405G. [8] D. Rehder, “The role of vanadium in biology” Metallomics, vol. 7, Pages 730–742, May 2015, https://doi.org/10.1039/c4mt00304g [9] N. Zhou, J. X. Zhang, M. T. Fan, J. Wang, G. Guo, and X. Y. Wei, “Antibiotic resistance of lactic acid bacteria isolated from Chinese Yogurts”, J. Dairy Sci. vol. 95, pp. 4775–4783, 2012. http://dx.doi.org/ 10.3168/jds.2011-5271 [10] 10. G. A. J. Ayliffe, W. Green, R. Livingston, and E. J. L. Lowbury, “Antibiotic-resistant Staphylococcus aureus in dermatology and burn wards”, J. Clin. Path., vol.30, pp. 40-44, 1977. [11] A. Patwardhan and J. A. Cowan, “Influence of charge and structure on the coordination chemistry of copper aminoglycosides” Dalton Trans., vol. 40, pp. 1795-1801, 2011 https://doi.org/10.1039/C0DT00704H [12] J. A. Obaleye and O. O. Abosede, “Cu(II) Complex of Lincomycin: Synthesis, Spectral Characterization and Competitive DNA Binding” Nigerian Journal of Chemical Research, vol. 24, pp. 15-19, 2019b. https://www.ajol.info/index.php/njcr/article/view/187649 [13] V. K. Srivastava, “Synthesis, characterization, and biological studies of some biometal complexes”, Futur J Pharm Sci, vol. 7, pp. 51 2021. https://doi.org/10.1186/s43094-021-00191-w [14] American Chemical Society National Historic Chemical Landmarks. Selman Waksman and Antibiotics. http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/selmanwaksman.html (accessed December 20, 2021).
Copyright
Copyright © 2022 Olufunso O. Abosede, Charles G. Ikimi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET39801
Publish Date : 2022-01-04
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

