Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Study of Physiological Parameters and Rheology for the Production and Extraction of Cyclosporin A
Authors: Prabhakar Babu, Yogesh Patel, Subhashis Pany, Ilma Majeed, Dr. Umesh Luthra
DOI Link: https://doi.org/10.22214/ijraset.2022.41268
Certificate: View Certificate
Abstract
This study is an evaluative effort to analyse different physiological parameters and rheological properties observed during the fermentative production of cyclosporin A. Tolypocladium inflatum culture was used for seed development. For production fermentation, the mature seed was inoculated at specific transfer parameters. At each stage of the current study, pH, PMV and sugar consumption were keenly analysed and recorded. Different aeration rates and agitation speeds were evaluated during various stages of growth. Based on several experiments, the most optimised parameters were included for further analysis. The maximum productivity obtained was 8.3 g/L. Further, different kinds of solvents were studied to extract crude from the fermentation broth. Solvents required at various stages of downstream processing were analysed for best recovery with minimal product loss. Finally, the product was obtained with 80.58% recovery.
Introduction
I. INTRODUCTION
Cyclosporin A (CyA) is chemically a cyclic undecapeptide which is lipophilic in nature [1] and a secondary metabolite that has been studied for different kinds of biological activities like anti-fungal and anti-parasitic nature [2], anti-inflammatory [3] and immunosuppressive properties [4]. Having received FDA approval for clinical use in 1983, CyA has now been used in regard of graft rejection prevention during transplantation of heart, kidney and liver [1], [5]. It has also been studied for several autoimmune disorders [6]. Obtaining CyA in substantial amount has been studied through several years of research as evident by literature studies. Different types of fermentations including submerged [7], solid state [8] and static [9], as well as enzymatic synthesis [10] have been reported. Aerobic fungi called as Tolypocladium inflatum is a renowned name in regard of industrial production of CyA [1], [7]. But the secondary metabolite has also been reported from species of Neocosmospora [1], Verticellium, Fusarium, Cylindrocarpon [11], [12] and several others. Different morphological characteristics and physiological parameters have been closely evaluated in association with enhanced production of cyclosporins. A study based on pigmented variants of Tolypocladium inflatum indicates the effect of colony morphology on productivity of the secondary metabolite [13]. Various optimisation techniques have also been reported in the context of high production [14]. Impact of medium components on the productivity is very well understood through modification of macronutrient sources [15]. With respect to the type of morphology shown by the fungal culture, studies indicate that pelleted form of growth is preferred over other morphological forms such as mycelial and clumps [7]. There is plethora of information about enhanced productivity of cyclosporin A by means of strain improvement, medium optimisation [1], [7], modification in minerals [8], [9] and nutrients added and environmental parameters [1], [8], [9]. But little amount of correlated data has been found with respect to changes in aeration, agitation rate and variations observed in pH, substrate consumption and metabolite production throughout the cycle.
In our study, we have investigated physiological parameters during seed and production fermentation using Tolypocladium inflatum. By varying the tip speed and modifying aeration rates, we have been able to record growth patterns and subsequent effect on production of cyclosporin A. The extraction and purification procedures undertaken have also involved several experiments in the quest of finalizing the most appropriate solvents during various stages of downstream processing.
II. MATERIALS AND METHOD
A. Seed Development
- Inoculum Preparation: Grown culture of Tolypocladium inflatum was scraped from the slants and mixed with 0.85% normal saline. Then, 2.0 mL of the suspension was transferred to the inoculum medium containing corn steep powder (20 g/L), dextrin white (20 g/L), dextrose (10 g/L), KH2PO4 (2 g/L) and MgSO4.6H2O (5 g/L). The culture was incubated at 27°C, 200 rpm for 72 hours. During this period, pH, Packed Mycelial Volume (PMV) were keenly analysed and once the culture attained maturity, with the transfer parameters being 5.0 pH and 12% PMV, it was transferred to inoculum transfer bottle and was eventually utilised to inoculate the seed fermenter medium.
- Seed Media: The fermenter employed for seed development was of 25 L with 16 L working volume. In order to prepare the seed media, 5.0 L of DM/RO water was taken in a container labelled as I. Water was heated to reach the temperature of 90 ?C and then 480 g of corn steep liquor and 240 g of dextrose were slowly added to it. The contents were mixed thoroughly and sterilized for 60 minutes. In another container labelled as II, distilled water was heated up to 90 °C. To it, were added, 64 g of MgSo4 and 32 g of KH2Po4. The contents of containers I and II were charged into the fermenter. Medium pH at this stage was 5.2, which was adjusted to 5.6 with the help of 20 % NaOH solution. Then, antifoam (Spectra UCN) was added to the fermenter. The media was sterilised at 121°C for 45 min and was then allowed to cool down to 28°C. Under stringent sterile conditions, the medium was inoculated with the prepared inoculum which was 1.5% of the seed fermenter medium volume.
- Seed Growth: The process parameters were strictly controlled in order to conclude best conditions for optimal growth of the organism. Throughout the process, temperature was maintained at 27°C. The effect of varying agitation and aeration conditions on the growth pattern was closely evaluated. Optimisation of the tip speed in the range of 1.5 m/s to 2.5 m/s was evaluated along with varying aeration rate between 0.5 to 1.0 vvm. Table 3 represents a correlation between the effects of different aeration and agitation rates on activity as well as various other parameters. During the entire process of fermentation, back pressure was around 0.5 – 1.0 Kg/cm2.
B. Fermentation
- Fermentation Media: In container I, 5.0 L of DM water was taken and heated up to 80-90 °C. To it, 2800 g of corn steep liquor was added and given heat shock at 90°C for 15 minutes. The container was allowed to cool down naturally for 2 hours, following which, it was sterilized in an autoclave at 121°C for 60 minutes separately. To 25 L RO/DM water heated up to 90°C, 7000 g malto dextrin, 350 g (NH4)2SO4, 175 g urea, and 1400 g sucrose were sequentially added. All the contents were mixed and the volume was made up using DM water. The pH at this stage was 4.5, which was then adjusted to 5.8 using 20% NaOH. Then, appropriate amount of antifoam (Spectra UCN) was added to the fermenter. The media was autoclaved at 121°C for 45 minutes.
- Fermentation Process: The mature seed of 44 hrs age and 18% PMV at 5.8 pH was transferred to the 100 L production fermenter with 70 L working volume. The percentage of seed transferred was 15 % (v/v). The process parameters were strictly monitored. Throughout the process, temperature was maintained at 27°C. For studying the effect of agitation speed on the process, variable amount of tip speeds were tested ranging between 2.8 m/s to 6.0 m/s. Aeration was varied between 0.5 to 1.5 vvm. During the entire process of fermentation, back pressure was around 0.5 – 1.0 kg/cm2.
???????C. Product Extraction and Recovery
Submerged fermentation was carried out for 240 hours. The fermentation broth obtained from the production fermenter was filtered and the cell mass extraction was performed using different solvents in order to assess the best one to be chosen for product extraction.
Each solvent, amounting to three times of the cell mass volume was mixed with the cell mass. The temperature was maintained at 30oC. After 3 hours of stirring process, the mixture was filtered and the concentrated mass from the most suitable solvent recognised by maximum purity and least product loss was taken for further process of concentration and precipitation.
For the purpose of precipitation of concentrated cell mass, we have employed different solvents and optimised the process using the most suitable one, with maximum recovery %.
Eventually, recrystallization of obtained precipitate was carried out for the final purification of cyclosporin A. Results at each step of extraction were tabulated and thoroughly analysed to obtain maximum product recovery.
III. RESULTS AND DISCUSSION
A. Effect of pH
Seed medium was inoculated with 1.5% inoculum and the evaluation of several factors was done continuously to analyse the maturity of seed. pH plays an eminent role during fermentation. According to a research conducted on implication of soil-derived T.inflatum, by Balakrishnan and Pandey [1, 16], the organism was found to endure pH range of 5 to 6. The study projected that when the final pH of culture was low, CyA was obtained in lower amount; while higher final pH led to enhanced production of the secondary metabolite. The table below tabulates information about pH changes during seed fermentation and the corresponding observations with regard to PMV.
TABLE 1
pH and pmv changes during seed fermentation
|
Age (Hrs.) |
pH |
PMV (%) |
|
0 |
5.4 |
- |
|
10 |
5.5 |
2 |
|
20 |
5.6 |
6 |
|
30 |
4.9 |
10 |
|
35 |
4.8 |
14 |
|
40 |
5.0 |
18 |
|
45 |
5.8 |
24 |
As the culture age was increasing, pH was found to increase continuously from 5.4 to 5.6. During this time, mycelium was found to grow at a slower pace. Once the adaptation phase of the cells was achieved, pH decreased to some extent and then finally it was found to increase to the maximum of 5.8. At this stage, the seed was mature enough (PMV=24%) to be transferred for production fermentation.
The results of production stage have been tabulated below:
TABLE 2
PRODUCTION FERMENTATION
|
Age (Hrs.) |
pH |
PMV (%) |
Activity (g/L) |
|
0 |
5.8 |
2 |
- |
|
12 |
6.0 |
8 |
- |
|
24 |
6.2 |
10 |
- |
|
48 |
6.4 |
12 |
1.3 |
|
72 |
6.6 |
15 |
2.1 |
|
96 |
6.8 |
22 |
3.4 |
|
120 |
6.0 |
28 |
4.7 |
|
144 |
5.6 |
32 |
6.3 |
|
168 |
5.1 |
36 |
6.8 |
|
192 |
4.9 |
40 |
7.0 |
|
216 |
4.6 |
44 |
7.8 |
|
240 |
4.9 |
42 |
8.3 |
Cyclosporin A was harvested between 200 to 240 hrs of fermentation process. The maximum productivity was found to be 8.3 g/L.
A comparison had been established between the morphological changes observed during seed and production fermentation. The figures below conglomerates the date obtained:
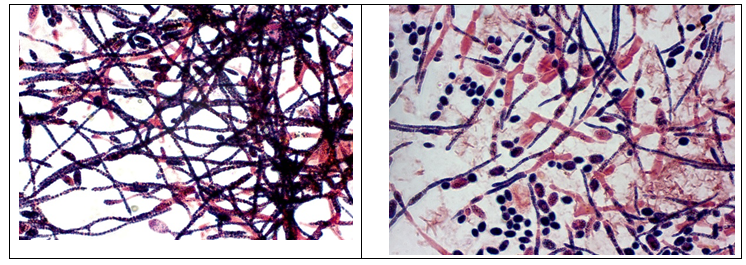
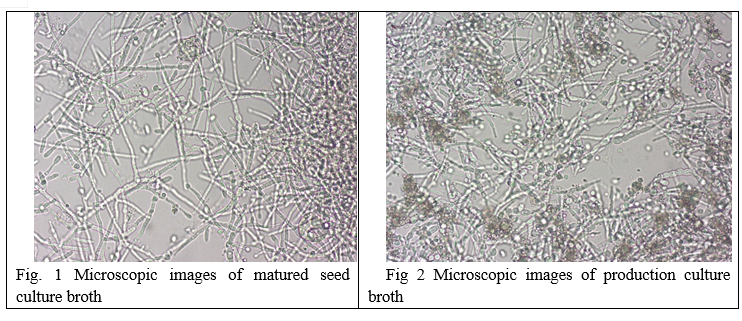
A mature seed culture, when studied under a light microscope, showed the growth of dense, long and branched network of mycelium with the presence of spores. Production culture broth was observed to show a dense growth of long mycelial network in the growth phase while sporulation was observed along with the network of mycelium during the production phase.
???????B. Effect of Aeration and Agitation
Aeration and agitation have an immense impact on overall mass and oxygen transfer [1] during submerged fermentation. Agitation or tip speed determines the rate at which nutrient and oxygen would be transferred within the fermentation liquid, whereas, aeration is responsible for the oxygen availability. They both help in determination of various rheological parameters, which are play a very important role in the study of cell kinetics.
During seed development, constant 1.5 m/s tip speed for 24 hours showed steady growth while gradually increasing it to 2.5 m/s, facilitated better absorption of nutrients and air throughout the medium, thus enhancing mycelial growth (Figure 1). At tip speed of more than 2.5 m/s, a reduction in growth with breakage of mycelia was observed. Studying a range of aeration possibilities between 0.5 to 1.0 vvm, finally the latter was found to be the most appropriate for the culture growth.
During fermentation in 100 L bioreactor, agitation was varied between 2.8 to 6.0 m/s. Liquid Dissolved Oxygen has been an eminent parameter to decide the tip speed of the fermenter. At a maximum of 6.0 m/s, DO% was between 30-40% which showed a steady mycelial growth with enhanced production of CyA.
The study was found to conglomerate well with the results of previous researches as conducted by El Enshasy et al. [11] and El-Refai et al. [17]. Controllable agitation and aeration in a fermenter effects growth of cells to a large extent and thereby metabolite production to a large extent.
In this investigation, total sugar content was also evaluated in various stages of growth. A graph comprising of the combined study has been included below:
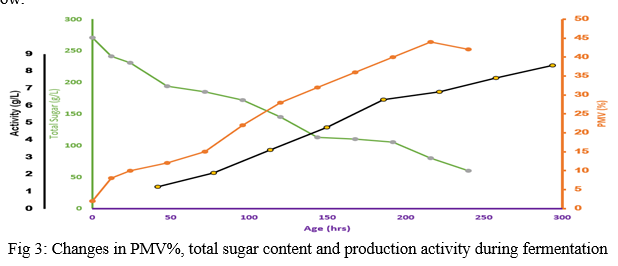
The above graph is a clear indication of the changes in key parameters during the entire phase of fermentative production of cyclosporin A.
???????C. Extraction and Recovery of Cyclosporin A
Over the years, several studies have been conducted on the methodology adopted to extract product in a way to obtain maximum purity [18]-[20]. The most common method to extract a metabolite from the fermented biomass using an organic solvent. The fermented broth is mixed with the solvent which is then distilled to obtain the residue.
The residue is reextracted, concentrated and finally subjected to various chromatographic techniques for precise purification as well as quantification.
- Solvent Extraction: For our study, we have employed several solvents to optimise the extraction of CyA. Ethyl acetate, toluene, MDC and acetone were separately used to obtain the concentrated cell mass from the fermentation broth. The results of 1 kg input cake obtained after filtration of the fermentation broth have been tabulated below:
TABLE 3
Solvent Selxction for Extraction
|
Solvent |
Volume (V) |
Temp |
Time |
Conc. mass |
Activity |
Actual Product |
Spent Cake |
Activity |
Product Loss |
|
Ethyl Acetate |
3V* 3T |
30 C |
3 |
54.4 |
470 |
25.57 |
0.75 |
2.04 |
1.53 |
|
Toluene |
3V* 3T |
30 C |
3 |
68.4 |
244.31 |
16.71 |
0.7 |
13.69 |
9.583 |
|
MDC |
3V* 3T |
30 C |
3 |
50.5 |
249.23 |
12.59 |
0.7 |
19.2 |
13.44 |
|
Acetone |
3V* 3T |
30 C |
3 |
87 |
283.92 |
24.70 |
0.6 |
2.43 |
1.458 |
Finally, we have used ethyl acetate for the extraction procedure because of high purity and minimum product loss. Though acetone also yielded substantial amount of product, but compared with the concentrated mass, purity obtained was very less.
2. Precipitation: After obtaining the concentrated mass, it was allowed to precipitate using various solvents. Here also, the implication of different solvents helped in understanding the effect on product recovery in the process of obtaining crude metabolite.
The results have been tabulated below:
TABLE 4
SOLVENT SELECTION FOR PRECIPITATION
|
Solvent |
Volume |
Temp (°C) |
Time |
Conc. mass (g) |
Activity |
Actual |
Crude |
Activity |
Actual |
Recovery |
|
Hexane |
130 |
0-5 |
6 |
13 |
470 |
6.11 |
6.7 |
712.5 |
4.774 |
78.130 |
|
Cyclo Hexane |
130 |
0-5 |
6 |
13 |
470 |
6.11 |
6.75 |
785.1 |
5.299 |
86.734 |
|
Heptane |
130 |
0-5 |
6 |
13 |
470 |
6.11 |
6.2 |
670.8 |
4.159 |
68.068 |
|
Hexane:Water (1:1) |
130 |
0-5 |
6 |
13 |
470 |
6.11 |
6.95 |
695.5 |
4.834 |
79.112 |
Among the different solvents used for obtained the precipitation of oil or concentrated mass, cyclohexane gave maximum percentage of product recovery with substantial amount of crude.
The crude obtained was recrystallized using acetone. Though ethyl acetate, methanol, ethanol and IPA were also tested in this regard but no crystallization was observed in any of them. The final results encompassing the product recovery percentage have been tabulated below:
Table 5
Recovery of Product
|
Solvent |
Volume |
Temp |
Time |
Crude |
Activity |
Actual Product |
Pure Product |
Activity |
Actual Product |
Recovery |
|
Acetone |
20 ml |
0-5 |
3 |
5 |
785.1 |
3.92 |
3.2 |
987.2 |
3.159 |
80.58 |
As seen from the results obtained, there was 80.58% of recovery while recrystallization of the precipitate.
IV. ACKNOWLEDGMENT
The authors wish to thank the management of Teyro Labs Private Limited for supporting this work. We also thank colleagues at Biotech department for their cooperation during this study
Conclusion
Tolypocladium inflatum culture was used for obtaining mature seed employable as inoculum for the production fermenter. Agitation and aeration rates play an important role in the growth of mycelia and resultant metabolite production. Varying ranges of the physiological parameters studied in the present investigation have helped in better understanding of the conglomerative factors\' interdependency. Finally, with optimised rates of agitation and aeration, increasing productivity has been observed. We have also been able to evaluate the changes in total sugar consumption throughout the fermentative study. Further, during downstream processing, we have been able to obtain maximum recovery with minimal product loss using optimisation of solvent and extraction conditions.
References
[1] Survase, S. A., Kagliwal, L. D., Annapure, U. S., & Singhal, R. S. (2011). Cyclosporin A—a review on fermentative production, downstream processing and pharmacological applications. Biotechnology advances, 29(4), 418-435. [2] Falah, F., Vasiee, A., Ramezani, M., Tabatabaee-Yazdi, F., Mortazavi, S. A., & Danesh, A. (2022). Effect of immobilization, mutation, and microbial stresses on increasing production efficiency of “Cyclosporin A”. Biomass Conversion and Biorefinery, 1-16. [3] Stellato, C., de Paulis, A., Ciccarelli, A., Cirillo, R., Patella, V., Casolaro, V., & Marone, G. (1992). Anti-inflammatory effect of cyclosporin A on human skin mast cells. Journal of investigative dermatology, 98(5). [4] Schreiber, S. L. (1991). Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science, 251(4991), 283-287. [5] Italia, J. L., Bhardwaj, V., & Kumar, M. R. (2006). Disease, destination, dose and delivery aspects of ciclosporin: the state of the art. Drug discovery today, 11(17-18), 846-854. [6] Utine, C. A., Stern, M., & Akpek, E. K. (2010). Clinical review: topical ophthalmic use of cyclosporin A. Ocular immunology and inflammation, 18(5), 352-361. [7] Anjum, T., & Iram, W. (2017). Production of Cyclosporine A by Submerged Fermentation. In Fungal Metabolites (pp. 783-810). Springer, Cham. [8] Survase, S. A., Shaligram, N. S., Pansuriya, R. C., Annapure, U. S., & Singhal, R. S. (2009). A novel medium for the enhanced production of cyclosporin A by Tolypocladium inflatum MTCC 557 using solid state fermentation. Journal of microbiology and biotechnology, 19(5), 462-467. [9] Survase, S. A., Annapure, U. S., & Singhal, R. S. (2009). Statistical optimization of cyclosporin A production on a semi-synthetic medium using Tolypocladium inflatum MTCC 557. Global J Biotechnol Biochem, 4(2), 184-192. [10] Ismaiel, A. A., El-Sayed, E. S. A., & Mahmoud, A. A. (2010). Some optimal culture conditions for production of cyclosporin a by Fusarium roseum. Brazilian Journal of Microbiology, 41(4), 1112-1123. [11] El Enshasy, H., Fattah, Y. A., Atta, A., Anwar, M., Omar, H., Magd, S., & Zahra, R. A. (2008). Kinetics of cell growth and cyclosporin A production by Tolypocladium inflatum when scaling up from shake flask to bioreactor. Journal of microbiology and biotechnology, 18(1), 128-134. [12] Irum, W., & Anjum, T. (2012). Production enhancement of Cyclosporin ‘A’by Aspergillus terreus through mutation. African Journal of Biotechnology, 11(7), 1736-1743. [13] Aarnio, T. H., & Agathos, S. N. (1990). Pigmented variants of Tolypocladium inflatum in relation to cyclosporin A production. Applied microbiology and biotechnology, 33(4), 435-437. [14] Dong, H., Jiang, J., Yan, T., & Zhao, J. (2011). Optimization of cyclosporin A production by Beauveria nivea in continuous fed-batch fermentation. Archives of Biological Sciences, 63(3), 907-914. [15] Tanseer, S., & Anjum, T. (2011). Modification of c and n sources for enhanced production of cyclosporin\'a\'by Aspergillus Terreus. Brazilian Journal of Microbiology, 42, 1374-1383. [16] Balakrishnan, K., & Pandey, A. (1996). Growth and cyclosporin a production by an indigenously isolated strain ofTolypocladium inflatum. Folia microbiologica, 41(5), 401-406. [17] EI-REFA?, H. A., ABD-EISAIAM, I. S., & Sallam, L. A. (2004). KINETIC STUDIES ON THE GROWTH AND CYCLOSPORIN A PRODUCTION BY A LOCAL ISOLATE OF FUSARIUM OXYSPORUMI, NRC. ACTA Pharmaceutica Sciencia, 46(3). [18] Ly, M., Margaritis, A., & Jajuee, B. (2007). Effect of solvent concentration on the extraction kinetics and diffusivity of Cyclosporin A in the fungus Tolypocladium inflatum. Biotechnology and bioengineering, 96(1), 67-79. [19] Sekar, C., & Balaraman, K. (1998). Optimization studies on the production of cyclosporin A by solid state fermentation. Bioprocess Engineering, 18(4), 293-296. [20] Balaraman, K., & Mathew, N. (2006). Optimization of media composition for the production of cyclosporin A by Tolypocladium species. Indian Journal of Medical Research, 123(4), 525.
Copyright
Copyright © 2022 Prabhakar Babu, Yogesh Patel, Subhashis Pany, Ilma Majeed, Dr. Umesh Luthra. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET41268
Publish Date : 2022-04-06
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

