Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Review: Controlled Release of Analgesics
Authors: Rashi Singh , Ashwini Bharati
DOI Link: https://doi.org/10.22214/ijraset.2023.49109
Certificate: View Certificate
Abstract
Abstract: The regulated release of medication into the patient provided by the patch makes transdermal drug delivery superior to other methods of pharmaceutical administration. The medication instantly enters the bloodstream through the skin. Low concentration in the blood and high concentration on the patch. The usage of polymers is successful in achieving analgesics\' sustained release. To evaluate effectiveness, in vitro and in vivo investigations were conducted. Due to the advantages that biodegradable polymers have over other materials in use, there is currently a huge demand for them. A biodegradable preparation of polymer and active ingredient is made for wound healing with a synthetic porous drug-loaded top layer and a spongy collagen sublayer. This method of analgesic delivery is slowly progressing among people and would further require few advancements as it is completely contrast to the conventional forms.
Introduction
I. INTRODUCTION
A. Novel Drug Delivery System (NDDS)
NDDS can be defined as new approach that combines innovative development, formulations, new technologies, new methodologies for delivering pharmaceutical composites in the body as demanded to safely achieve its asked pharmacological goods.
Several types of NDDS have been developed during last many decades which are- Microparticles, Nanoparticles, Osmotically Modulated Drug Delivery Systems, Transdermal Therapeutic Systems (TTS), Aquasome, Dendrimers, Multiple Emulsion, Microemulsions, Liposomes, Niosomes, Pharmacosomes, Self-Regulating Drug Delivery System, Brain Targeted Delivery System etc.
B. Controlled Drug Delivery System (CRDDS)
Controlled medicine Delivery is the one which delivers the drug at a predetermined rate, for locally or systemically, for a specified period of time.
There are basically two types of drug delivery systems:
- Conventional drug delivery system
- Novel drug delivery system
In Conventional drug delivery system, the state-of-the-art methodologies of medicine delivery include the preferred non-invasive peroral routes, the topical route, the transmucosal route and inhalation route.
As Conventional delivery causes fluctuations in plasma levels due to immediate release. Thus, to maintain the therapeutic window of medicine attention it is needed to adopt new drug delivery system.
Novel drug delivery helps in administering the specific medications such as peptides, proteins, antibodies, vaccines, gene-grounded drugs without causing enzymatic declination and furnishing efficient bioavailability, etc which cannot be achieved when administered via conventional routes.
Controlled drug delivery has a number of benefits that are now widely acknowledged. One benefit is their ability to continuously distribute the active drug at a constant rate over an extended period, without action being delayed and without interference from gut physiology or other external factors. Advantage is that is improves patient compliance and lowers the likelihood of side effects due to time between doses is longer. The chemically-controlled systems are one of these cutting-edge medication delivery methods.
Drugs are incorporated into a biodegradable polymer for controlled release. The medication is released as result of chemical reaction, travels to the site of action and the polymer that is left is degraded in the body without the need to concentrate on getting the drug out of the body.
Pain is a physiological phenomenon that helps prevent damage from a harmful stimulus or detect the presence of a disease or injury. When a patient cannot tolerate the pain experienced, analgesic drugs are used to achieve pain relief. These drugs are needed for long-term, however, their analgesic effects typically have short duration and serious side effects.
To address these issues, polymers that contain analgesic drugs chemically incorporated within the polymer backbone or as pendant groups were designed, synthesized, characterized, and formulated.
Patient-controlled analgesia include self-delivery of pain medication, faster alleviation of pain because the patient can address pain with medication, and dosage monitoring by medical staff (dosage can be increased or decreased depending on need). With a PCA the patient spends less time in pain and as a corollary to this, patients tend to use less medication than in cases in which medication is given according to a set schedule or on a timer.[1] [5]
C. Types Of Pain Killers
Multiple painkillers are typically used to treat post-surgical pain (analgesics). Depending on the type of surgery and anticipated recovery, as well as your particular needs, the right prescription for you will depend on delivery method and dosage.
Medications include the following:
- Opioids, potent painkillers that reduce the impression of pain, may be administered following surgery. Fentanyl, hydromorphone, morphine, oxycodone, oxymorphone, and tramadol are examples of intravenous opioids. Following surgery, oxycodone (OxyContin, Roxicodone, among others) and oxycodone with acetaminophen are a few examples of opioids that may be provided as pills (Percocet). Lidocaine and bupivacaine are two examples of local anaesthetics that temporarily numb a specific area of the body.
- Nonsteroidal anti-inflammatory medicines (NSAIDs), such ibuprofen (Advil, Motrin IB, etc.), naproxen sodium (Aleve, Anaprox DS, etc.), celecoxib (Celebrex), or ketorolac, reduce the inflammatory activity that makes pain worse. Other nonopioid pain relievers include acetaminophen (Tylenol, others) and ketamine (Ketalar).
- Midazolam, an anti-anxiety medicine, or the anticonvulsants gabapentin (Gralise, Horizant, Neurontin), and pregabalin, anticonvulsants, may also be used to alleviate post-surgical pain (Lyrica).
While using opioids after surgery may or may not be acceptable, your doctor will probably recommend a combination of remedies.
Skin can be divided into three main regions: (1) the outermost layer, the epidermis, which contains the stratum corneum; (2) the middle layer, the dermis and (3) the inner most layer, the hypodermis.
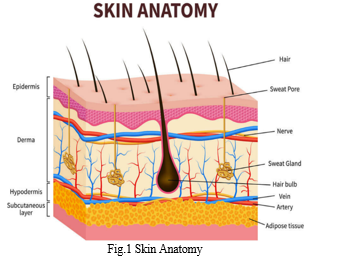
The development of effective TDD systems require knowledge of the kinetics of skin permeation.
a. Penetration: The entry of a substance into a particular layer of the skin;
b. Partitioning from the stratum corneum into the aqueous viable epidermis;
c. Diffusion through the viable epidermis and into the upper dermis;
d. Permeation: The penetration of molecules from one layer into another, which is different from the first layer both functionally and structurally.
e. Absorption: The uptake of a drug into the systemic circulation. iii. Diffusion through the functional epidermis and into the upper dermis. [2]
II. MATERIAL AND METHOD
A. Transdermal Route
TDD is a painless method of systemic drug delivery that involves applying a drug formulation to healthy, undamaged skin. Without building up in the dermal layer, the medication first goes through the stratum corneum and then deeper layers of epidermis and dermis.
Enhancing Transdermal Drug Delivery Through:
- Thickness, Weight Variation, and Drug Content: The thickness of the patch was measured using a thickness gauge at three distinct positions, and the weight of each patch removed from the cast film was then calculated using a digital scale. Ten randomly chosen patches were weighed individually to subject the patches to weight fluctuation
- A mixture of chemical substances or components known as a eutectic system has a single chemical composition that solidifies at a lower temperature than any other composition. Regular solution theory states that a substance, including skin lipids, is more soluble in a given solvent the lower its melting point.
- Liposomes and vehicles: Drug-encapsulating liposomes are colloidal particles generated as concentric bimolecular layers. There are numerous instances of cosmetic goods with vesicles encasing the active components. Although many other potential constituents have been considered, the most typical composition is phosphatidylcholine from egg or soybean yolk.
- Iontophoresis: This technique entails a low-level electric current being administered to the skin, either directly or indirectly through a dosage form, to permeate a topically applied medicinal substance. The electrode type, current intensity, and pH of the system are variables that affect the design of an iontophoretic skin delivery system.
- Laser radiation and photomechanical waves Lasers are widely used to rejuvenate the face and cure dermatological disorders like acne. This procedure includes applying a laser to the skin in a direct and regulated manner. This eliminates the stratum corneum while barely affecting the epidermis beneath.
- Radio frequency: This method exposes skin to high-frequency alternating current, which causes the membrane to generate heat-induced microchannels.
- Membrane Permeation–Controlled Systems: In this kind of system, the drug reservoir is enclosed in a shallow compartment made of a laminated metallic plastic that is impermeable to drugs and a polymeric membrane that controls the rate of permeation and may be either microporous or non-porous. Only the rate-regulating polymeric membrane allows the medication molecules to release.
- Formulation Of The Patch: The patch's composition consists of three substrates linked together by two layers of glue that contains a medication. Before being incorporated into the product, the medicine must first be processed into the physical or chemical form needed. After that, a solvent is combined with the excipients and drug adhesive components to create a homogeneous solution. On moving objects, these adhesive formulations are applied as a thin film, which is then dried to eliminate the solvent. The five-layer product, which consists of a release linear contact adhesive control membrane, a drug reservoir, and a backing substrate, is then created by laminating the dried adhesive film with additional layers. The final dosage form was then printed on the lamination and die-cut. After that, the product is packaged in individual foil packets.[3]
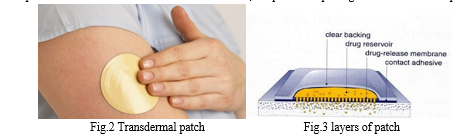
B. Polymeric Method
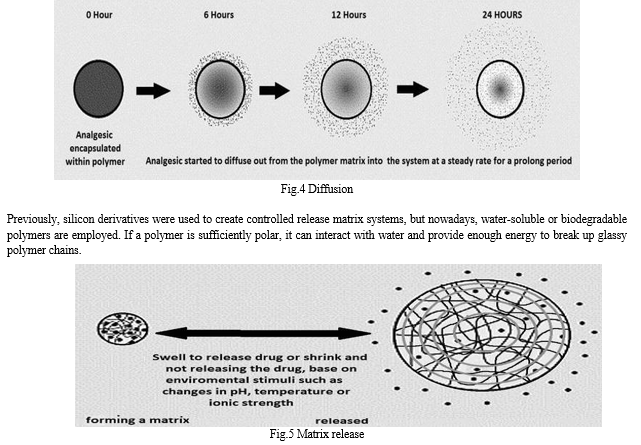
Salicylic acid (SA), ibuprofen, naproxen, and opioids like morphine and nalbuphine were polymerized to create drug delivery systems with the potential to prolong the effects of analgesics, lessen adverse effects, and avoid unintentional drug withdrawal or overdose. The therapy of chronic inflammatory illnesses that produce pain may benefit from prolonged (i.e., weeks and months) NSAID release, and controlled opioid release may enhance the management of chronic pain. Microspheres made of poly(anhydride-ester) based on salicylate (PAE) were created as injectable delivery devices for sustained release of SA.
In order to alter the physicochemical characteristics and drug release patterns to achieve long-term SA release, the usage of copolymers and polymer blends was investigated. The polymer needs to be sterile in accordance with commercial and pharmacopeial standards. Thus, the impact of gamma and electron radiation on the physicochemical characteristics of the salicylate-based PAEs was investigated. A PAE backbone was chemically integrated with morphine. The "PolyMorphine" polymer was created, produced, and thoroughly characterised. Through in vitro research, the polymer's hydrolytic degradation mechanism was identified. Studies conducted in vitro showed that PolyMorphine is not harmful to fibroblasts. In vivo tests on mice revealed that PolyMorphine has a 20-fold longer analgesic window. Tartaric acid, 1,8-octanediol, and ibuprofen or naproxen as pendant groups make up a novel biodegradable polyester that was created and described. The polymers controllably and without burst release the free medication (ibuprofen or naproxen) in vitro. The medications remain bioactive after being freed from the polymer, and these novel biomaterials are not harmful to mouse fibroblasts or macrophages produced from human blood.[4]
C. Novel Bioresorbable Hybrid Structure Of For Wound Healing
Creation and research of bioresorbable hybrid structures that combine a collagen sublayer with synthetic pores that are drug-loaded on top. Ibuprofen or bupivacaine were put into the top layer, which was made utilising the freeze-drying of inverted emulsions approach, for controlled release to the wound site. The parameters of the emulsion were investigated for their influence on the microstructure, the ensuing drug-release profile, and the physical and mechanical properties. The semi-occlusive top layer's shape not only greatly influences the drug release profile but also allows control over vapour transmission. The duration of the analgesic medications' release ranged from a few days to over 100 days. Higher organic: aqueous phase ratios and polymer contents slow down both medications' burst releases and extend them because of poorer porosity. The mechanical qualities of this layer were enhanced by the inclusion of reinforcing fibres. Due to our unique preparation technique, good binding of the two components, PDLGA and collagen, was accomplished.[5]
III. DISCUSSION
Although drugs administered in transdermal dosage forms are typically poorly absorbed, this has the advantage of creating a dose form with a tightly controlled depot effect. The medicine may produce focused irritation, which is a common issue, even though it is the best dose form for analgesics. Currently, only a very small number of medicines, those with low molecular weights and high lipophilicity, are available on the market for transdermal drug delivery for systemic effects. Controlled medication release for a stable plasma profile may be adjusted for transdermal drug delivery systems. Transdermal dose forms may give doctors the chance to give their patients more therapeutic options in order to improve their care. Our knowledge of the stratum conium barrier's characteristics and how chemicals interact with and affect this structure has improved in recent years as a result of the application of several biophysical approaches.[6]
Controlled drug delivery formulations and the polymers utilised in these systems have advanced significantly in recent years, enabling them to perform functions more than merely extending a medication's effective release period. For instance, modern controlled-release systems can react to modifications in the biological environment and deliver—or stop delivering—drugs in accordance with these modifications. Materials that could eventually lead to targeted delivery systems have also been created. With these systems, a specific formulation could be guided to the particular cell, tissue, or place where the medicine it contains is to be administered. Emerging technologies provide opportunities that scientists have only just begun to investigate, despite the fact that much of this work is still in its early stages.
Natural polymer:
Polysaccharides- Protein based polymer-
- Alginate 1) Gelatin
- Cyclodextrin 2) Albumin
- Chitosan 3) Collagen
- Dextran
- Agarose
- Hyaluronic acid
- Starch
- Cellulose
Polylactic acid, polyglycolic acid, polyhydroxyl butyrate, polyester, and PLGA are examples of synthetic polymers.
a. Polyadepic acid, polysebacic acid, and polyterpthalic acid are polyanhydrides.
b. Polyamino acid: polyamide
c Polymers made of phosphorous, such as polyphosphates, polyphosphonates, and polyphosphazenes
Hydrogels are polymers that will expand without dissolving when introduced in water or other biological fluids, and they make up the majority of the materials utilised in swelling-controlled release systems. These hydrogels have a high absorption capacity and, at equilibrium, typically contain between 60 and 90 percent water and just 10 to 30 percent polymer.[7]
In a perfect world, a wound dressing would keep the wound surface moist, permit gas exchange, serve as a barrier against microorganisms, and drain excessive exudates. Development and investigation of bioresorbable hybrid structures that combine a synthetic, porous drug-loaded top layer with a spongy collagen sublayer are necessary to give these properties.[8]
Conclusion
The medication distribution technique is a significant and critical area of research and development for novel analgesic products. Additionally, transdermal administration offers a convenient method for taking drugs for a long period, which is beneficial in the control of pain. An improved level of safety may be offered by transdermal delivery for drugs having a narrow therapeutic window.[9] Controlling the drug distribution for analgesics has the dual goals of achieving more effective treatments while removing the risk of both under- and overdose. The level of safety awareness is increasing, and at that point, localisation of the goal becomes crucial. Since this is one method of introducing new goods into the controlled market, NDDS is also preferred in the new patent regime. Numerous similar items are offered on the Indian market as a result of extensive research being conducted there in both the public and commercial sectors.[10]
References
[1] https://en.wikipedia.org/wiki/Patient-controlled_analgesia#cite_note-Merck-5 [2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4695828/#:~:text=2.-,Transdermal%20Drug%20Delivery%20 (TDD) accumu lation %20in %20the %20 dermal%20layer [3] http://publications.anveshanaindia.com/wp-content/uploads/2018/05/FORMULATION-DEVELOPMENT-AND EVALUATIO N-OF-CONTROLLED-DRUG-DELIVERY-OF-ANALGESICS-VIA-NOVEL-ROUTES.pdf [4] https://rucore.libraries.rutgers.edu/rutgers-lib/39669/ [5] https://pubmed.ncbi.nlm.nih.gov/24316366/ [6] http://publications.anveshanaindia.com/wp-content/uploads/2018/05/FORMULATION-DEVELOPMENT-AND-EVALUATIO -OF-CONTROLLED-DRUG-DELIVERY-OF-ANALGESICS-VIA-NOVEL-ROUTES.pdf file:///C:/Users/rsing/OneDrive/ Desktop/(PDF)%20Advance% 20Delivery%20System%20D osage%20Form%20for%20Analgesic,%20Their%20Rationale,%20and%20Specialty.html [7] https://www.mddionline.com/news/polymers-controlled-drug-delivery [8] https://pubmed.ncbi.nlm.nih.gov/24316366/ [9] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5488073/ [10] https://www.researchgate.net/publication/228893742_Current_Status_and_Future_Prospects_of_New_Drug_Delivery_System
Copyright
Copyright © 2023 Rashi Singh , Ashwini Bharati . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET49109
Publish Date : 2023-02-14
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

