Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Synthesis, Characterization and Catalytic Applications of Chitosan Based Schiff Base Complexes of Copper (II)
Authors: Rose Mary, Aleena Xavier, Namitha Romeo
DOI Link: https://doi.org/10.22214/ijraset.2022.47037
Certificate: View Certificate
Abstract
Chitosan Schiff Base was synthesized using conventional method and its corresponding metal complex was synthesized using Copper sulphate crystals. CSB and the metal complex were characterized using analytical and spectroscopic techniques like FT-IR and SEM. The excellent catalytic activity of the synthesized CSB-Cu complex was studied with Knoevenagel Reaction.
Introduction
I. INTRODUCTION
A. Schiff Base
Schiff bases were first reported by Hugo Schiff in the year 1864.Schiff base is the condensation product which forms when a primary amine reacts with a carbonyl compound. It has got a general formula of R2C=NR′[Anant and Devjani,2011]. They can be considered as a subclass of imines being either secondary ketimines or secondary aldimines depending on their structure. Schiff base is an important class of ligands due to their synthetic flexibility, their selectivity and sensitivity towards the central metal atom and structural similarities with natural biological substances.
It has a wide range of applications mainly in medical and pharmaceutical fields. The schiff base and its metal complexes are very important catalysts in various biological systems, polymers, dyes, medicinal and pharmaceutical fields and these are used in birth control, food packages and as an oxygen detector.
B. Chitosan
Chitosan is a biopolymer derived from chitin and is the second most abundant polymer. It is made by treating chitin shells of shrimps and other crustaceans with an alkaline substance like sodium hydroxide [Wikipedia]. When it comes to the structure, chitosan is a linear polysaccharide.
- Applications Of Chitosan: This polymer has gained importance in numerous fields like water engineering, cosmetics, paper industry, textile industry, food industry, agriculture, photography, chromatographic separations and solid state batteries. When it comes to biomedical applications, chitosan is extensively used in tissue engineering, burn treatment, artificial skin, wound healing, wound dressing, ophthalmology and drug delivery systems. This is attributed to the presence of highly reactive hydroxyl groups and amino groups present in chitosan. This compound is extensively used in manufacture of shampoos, rinses, hair colorants, hair sprays and hair tonics. Several derivatives of chitosan and chitin have potential applications in hair care [P Dutta et al,2004].
C. Chitosan Schiff Base
It can be modified into many derivatives through physical and chemical processes. In this work, chitosan was converted to Chitosan Schiff Base (CSB) by bringing about a condensation reaction between chitosan and salicylaldehyde along with the elimination of water molecule.
Biological applications of CSB include antimicrobial activity, anticancer activity, antioxidant activity, drug carrier ability and tissue engineering capacity [P Dutta et al,2004].They can be easily prepared and it acts as an excellent chelating agent. But despite of having these mentioned advantages, it also has serious demerits which limit its use to basic conditions as they are insoluble in aqueous medium and it decomposes in acidic conditions [Supriya et al,2017].
D. Chitosan Schiff Base – Cu(II) Complex
The synthesized chitosan schiff base was used to make chitosan schiff base metal complex. This was accomplished by working out a reaction between CSB and copper sulphate crystals (anhydrous).They are able to show excellent catalytic activity in various reactions and in the presence of moisture. This feature of schiff base complexes is attributed to moisture stability [Anant and Devjani,2011]. Generally elemental analysis data may confirm the formation of chitosan schiff base as well as the coordination reaction of CSB with copper ions.
E. Knoevenagel Reaction
The Knoevenagel condensation reaction is an organic reaction named after German chemist Emil Knoevenagel. It is the modified reaction of the Aldol condensation. A knoevenagel condensation is a nucleophilic addition for an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence condensation). The product is often α,β-unsaturated enone. In this reaction, the carbonyl group is an aldehyde or a ketone and the catalyst is usually a weakly basic amine. Nowadays Knoevenagel condensation reaction is one of the basic reactions in organic chemistry, for its significant synthetic utility in carbon- carbon bond formation, which is pivotal in organic synthesis [Gadekar and Lakshman,2010]. In order to establish the catalytic activity of CSB-Cu complex, Knoevenagel reaction was selected.
- Applications Of Knoevenagel Reaction: The Knoevenagel condensation is a key step in the commercial production of the antimalarial drug, lumefantrine. It is used in the synthesis of conjugated enones which serve as key intermediates in various reactions. The Hantzsch pyridine synthesis, the Gewald reaction and the Feist-Binary furan synthesis, all contain a knoevenagel reaction step. The reaction also led to the discovery of 2-Chlorobenzalmalononitrile (CS gas) [K Bodapati,2014]. It is also employed in the synthesis of therapeutic drugs, natural products, herbicides, insecticides, fine chemicals, in the synthesis of important intermediates or end product for perfumes, pharmaceuticals, calcium antagonists and polymers. It is also useful for further transformations, such as Diels-Alder and Michael additions [Gadekar and Lakshman,2010].
II. MATERIALS AND METHODS
A. Materials
Chitosan, Salicylaldehyde, Acetic acid, NaOH pellets, Malononitrile, Methanol were manufactured by Nice Chemicals (P) Ltd, Kochi, Kerala.
B. Experimental Methods
- Synthesis of Chitosan Schiff Base: A solution of Chitosan was made by dissolving 1g chitosan in 25ml of 2% acetic acid. To this solution, 1g NaOH pallets dissolved in 10ml water was added to swell. To this solution, 1ml Salicylaldehyde dissolved in 15ml of methanol solution was added.It was stirred on the magnetic stirrer for 1 hour. A yellow coloured solid of Chitosan Schiff Base was formed. It was dried for 30 minutes at 60° C. 0.4718g of chitosan schiff base was yielded.
- Synthesis of copper(II) complex of chitosan Schiff base: 0.0638g of synthesized Chitosan Schiff Base was stirred in 25ml ethanol for 1 hour. 0.2g of Copper Sulphate was dissolved in 10ml ethanol and stirred for 1 hour. Both the stirred solutions are mixed and stirred again for one and a half hours. It was filtered and washed using ethanol solution. The obtained green coloured solid was dried. 0.3g of CSB-Cu (II) complex was yielded.
- Knoevenegal Reaction using Schiff base metal complex: 5 millimoles of salicylaldehyde and 5 millimoles of malononitrile were mixed together along with 5 ml methanol and 0.3g of chitosan schiff base metal complex was added and continuous stirring result in product formation.
C. Characterization
- Fourier Transform Infrared Spectroscopy (FT-IR): Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) involves the interaction of infrared radiation with matter. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic techniques, it can be used to identify and study chemical substance. Samples may be solid, liquid or gas. The method or technique of IR spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) to produce an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency or wavelength on the horizontal axis. Typical units of frequency used in IR spectra are reciprocal centimeters (sometimes called wave numbers), with the symbol cm-1.Units of IR wavelength are commonly given in micrometers (formerly called “micron”), symbol μm, which are related to wave numbers in a reciprocal way. A common laboratory instrument that uses this technique is a Fourier transform infrared spectrometer. Two- dimensional IR is also possible as discussed below. The infrared portion of the electromagnetic spectrum is usually divided into three regions; the near-, mid- and far-infrared, named for their relation to the visible spectrum. The higher-energy near-IR, approximately 14000-4000cm-1 (0.7-2.5μm wavelength) can excite overtone or harmonic molecular vibrations. The mid-infrared, approximately 4000-40 cm-1 (2.5-25 μm) may be used to study the fundamental vibrations and associated rotational- vibrational structure. The far-infrared, approximately 400-10cm-1 (25-1000 μm), lying adjacent to the microwave region, had low energy and may be used for rotational spectroscopy. The names and classifications of these subregions are conventions and are only loosely based on the relative molecular or electromagnetic properties [Wikipedia]. The stretching frequencies of Chitosan Schiff Base and Chitosan Schiff Base-Cu (II) Complex were examined by Fourier Transform Infrared Spectroscopy using SAIFFT190927B.
- Scanning Electron Microscopy (SEM): A scanning electron microscope (SEM) is a type of electron microscope that produces images of a sample by scanning the surface with a focused beam of electrons. The electrons interact with atoms in the sample, producing various signals that contain information about the surface topography and composition of the sample. The electron beam is scanned in a raster scan pattern, and the position of the beam is combined with the intensity of the detected signal to produce an image. In the most common SEM mode, secondary electrons emitted by atoms excited by the electron beam are detected using an Everhart-Thornley detector. The number of secondary electrons that can be detected and thus the signal intensity depends among other things on specimen topography. SEM can achieve resolution better than 1nm. Specimens were observed in high vacuum in conventional SEM or in low vacuum or wet conditions in variable pressure or environmental SEM and at a wide range of cryogenic or elevated temperatures with specialized instruments [Stokes,2008]. The Scanning Electron Microscopic (SEM) images of Chitosan Schiff Base and Chitosan Schiff Base-Cu (II) Complex were obtained using JSM- 6390.The SEM images were used for the study of surface morphology.
III. RESULTS AND DISCUSSION
0.4718g of chitosan Schiff base and 0.3g of CSB-Cu(II) complex were yielded.
A. Fourier Transform Infrared Spectroscopy
The FT-IR spectrum was taken in order to illustrate the intra molecular interactions between the various components of synthesized CSB and CSB metal complex.
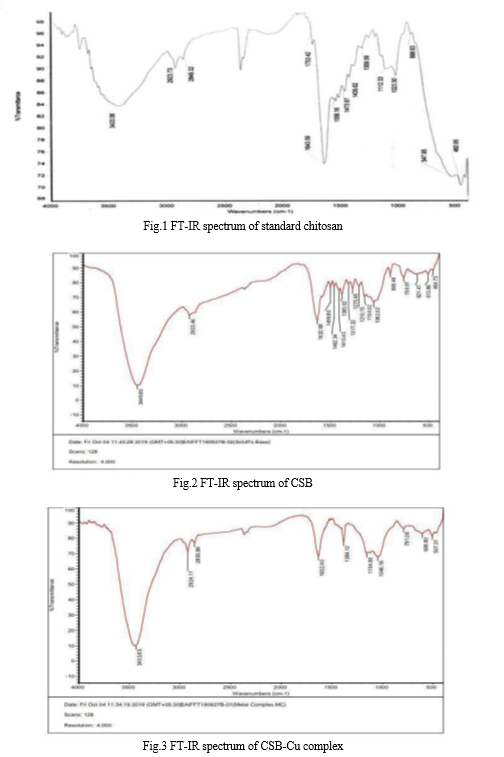
The FT-IR spectra of chitosan, CSB and CSB metal complex are shown in fig.1, fig.2 and fig.3 respectively. The comparative spectral details of chitosan, CSB and CSB metal complex are given in Table 1.
The FT-IR spectrum of chitosan(fig.1)[Google img.] shows a strong band at 3420cm-1 analogous to the O-H stretch which mask the characteristic band for the N-H stretch of amino group, while the axial stretching of C-H group is noted at 2849cm-1. The characteristic asymmetric -C-O-C- bridge stretching band occur at 1023.30cm-1, due to skeletal vibration of -C-O- stretching and bands of the β-(1-4) glycoside bridge monitored at 1112.30cm-1 are characteristic of chitosan saccharide structure.
For CSB (fig.2), the broad peak which was obtained at 3449.85cm-1.It indicates the presence of –OH hydroxyl and –NH stretching vibration. The peaks which were observed at 2923.46cm-1 proves the presence of asymmetrical C-H stretching in –CH group and aldehydic C-H stretching vibration.
A strong absorption band which was obtained at 1632.68cm-1 concludes the presence of C=N stretching (Schiff base) formed due to chitosan salicylaldehyde interactions. A peak at 1499.80cm-1 corresponds to aromatic C=C stretching and a peak at 1462.34cm-1 corresponds to N-H bending. In addition, the presence of functional groups such as C-O stretching in alcohol, C-O-C linkage, C-H out of plane deformation and C-C bending of chitosan salicylaldehyde schiff base were confirmed by the appearance of peaks at 1154.02cm-1, 1062.02cm-1, 895.48cm-1, 464.73cm-1 respectively.
In the FT-IR spectra of the CSB metal complex (fig.3), it is expected that coordination of nitrogen centre to the metal ion would reduce the electron density in the azomethine link and shift the C=N stretching frequency to the lower wave number. The shift in C=N stretch to the lower region was found in the spectrum of the CSB metal complex which shows the successful coordination of azomethine nitrogen to the metal centre. In the case of metal complex, the band in the region 606.50cm-1 and 507.31cm-1 were attributed to M-O and M-N stretching vibration confirming to the coordination to schiff base to metal ion.
|
Compound |
υ (C=N) cm-1 |
υ (O-H) cm-1 |
υ (M-O) cm-1 |
υ (M-N) cm-1 |
|
Chitosan |
1643.59 |
3420.06 |
- |
- |
|
Chitosan- Salicylaldehyde Schiff Base |
1632.68 |
3449.85 |
- |
- |
|
Cu-Schiff Base Complex |
1632.40 |
3433.63 |
606.80 |
507.31 |
Table 1: FT-IR Spectral analysis of Chitosan, Chitosan Schiff Base and its metal complex
B. Scanning Electron Microscopy
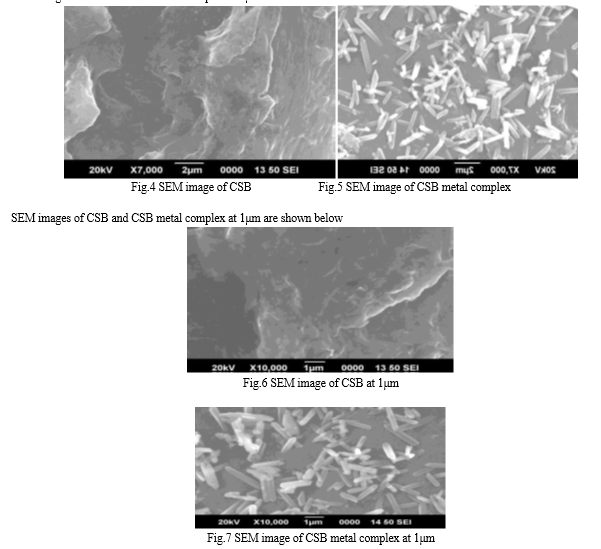
SEM images were taken for the study of surface morphology of CSB and its metal complex. SEM image of CSB (fig.4) had surface morphology smooth and uniform. The surface morphology of CSB was changed in CSB metal complex (fig.5). It had become uneven and aggregated in CSB metal complex.
SEM images of CSB and CSB metal complex at 2μm are shown below:
C. Knoevenagel Reaction
The reaction was carried out in required ambient conditions as provided in the procedure. This metal complex of chitosan based Schiff base was found to act as catalyst in Knoevenagel reaction as it remarkedly reduced the time required for completion of reaction. Due to inherent advantages of heterogeneous catalysis over homogeneous catalysis, a great deal of efforts has been devoted to the development of heterogeneous catalyst. Chitosan based Schiff base metal complex which acts as a heterogeneous catalyst catalyze the Knoevenagel reaction. Thus chitosan based Schiff base metal complexes has a major role in catalytic activity.
Conclusion
Synthesis of schiff base from salicylaldehyde with chitosan was carried out. The synthesized Schiff base ligand has been successfully complexed with the metal ion Cu. Characterization of the above schiff base and its metal complex were carried out. The FTIR spectral data of the ligand showed a band at a region of 1580-1680 cm-1, which is assigned to C=N stretching frequency, a feature of schiff base. This band is also observable in complexes, suggesting the ligand has coordinated to the metal. In the case of complexes, the band in the region 606.50cm-1 and 507.31cm-1 were attributed to M-O and M-N stretching vibrations respectively, conforming coordination of schiff base to metal ion. Surface morphology of the schiff base and the metal complexes have been examined using SEM. Metal complexes showed porous region compared to the parent ligand which may arise from the contraction of the voids by the cooperative contribution of ligand for complexation with metal ions. This gave a further evidence for complexation. The catalytic activity of the metal complex was analyzed based on Knoevenagel reaction. Chitosan based schiff base metal complex which acts as a heterogeneous catalyst catalyze the Knoevenagel reaction. Hence, chitosan based schiff base metal complex acts as good catalyst and has significant applications in altering rate of chemical reactions.
References
[1] Anant Prakash and Devjani Adhikari (2011) : Application of Schiff bases and their metal complexes- A Review. [2] Gadekar and Lakshman Sandurao (2010) : Modification characterization and applications of naturally occurring molecular sieves. [3] Kumar Bodapati (2014): Knoevenagal Reaction. [4] Pradip Kumar Dutta, Joydeep D and Tripathi VS (2003): Chitin and chitosan: chemistry, properties and applications. Journal of scientific and Industrial Research. [5] Stokes, Debbie J (2008) : Principles and practices of Variable Pressure Environmental Scanning Electron Microscopy (VP- ESEM). Chichester: John Wiley & Sons. [6] Supriya Prasad, Thandapani Gomathi and P. N. Sudha (2017) : Synthesis and Characterization Chitosan Schiff Base (CSB) and its Polyethylene Glycol Blend. [7] Wikipedia.
Copyright
Copyright © 2022 Rose Mary, Aleena Xavier, Namitha Romeo. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET47037
Publish Date : 2022-10-10
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

