Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- References
- Copyright
Synthesis of 1, 2 Disubstitued Benzimidazoles using SiO2/CaCl2.2H2O as a Catalyst
Authors: Jyoti Pandey Tripathi, Virendra Kumar Kasanaa
DOI Link: https://doi.org/10.22214/ijraset.2023.54392
Certificate: View Certificate
Abstract
disubstituted bezimidazole derivatives have been synthesized with excellent yield using SiO2/CaCl2.2H2O as a catalyst with o-phenylenediamine and various aldehydes by convenstional as well as microwave irradiation method. The advantage of this synthetic method i.e. greener protocol, easy handling and commercially available inexpensive catalyst.
Introduction
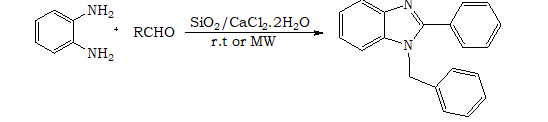
I. INTRODUCTION
1, 2-Disubstituted benzimidazoles are an important class of heterocyclic compounds that exhibit diverse biological activities and have applications in medicinal chemistry and materials science. Several synthetic methods have been developed to access these compounds, and catalytic systems play a crucial role in achieving efficient and selective transformations. In recent years, SiO2/CaCl2·2H2O has emerged as a promising catalyst for the synthesis of 1,2-disubstituted benzimidazoles due to its excellent catalytic activity, low cost, and easy availability.
In organic synthesis, having the lots of the biological activity and numerous natural products heterocyclic compounds are very important (1). Benzimidazole are a important class of heterocyclic compounds which have a charaterstic fusion of six membered benzene ring and five membered imidazole ring. Due to this fusion benzimidazole has an important pharmacophore. It have immense importance in medicinal and drug development area due to its privileged structure. Naturally occurring biologically active substances such as Vitamin B12 and purine bases include magical benzimidazole nucleus in their Structure (2). Benzimidazoles are one of the most biologically active class of compounds, having wide spectrum of activity. It plays an important role with Numerous therapeutic activities such as antiulcer (3), anti-inflammatory, analgesic (4-6), anti-fungal (7), anti-microbial (8-9), antihelminthic (10), anti-cancer (11), antihistaminic compounds (12-13) antiviral derivatives, anticonvulsant (14,15) etc. They are also inhibitors of photosynthesis, aldose reductase and antagonist of neurotransmitter receptors and They also are found to exhibit appreciable herbicidal activity (16).
Most of the described methods uses volatile organic solvent and used solid phase synthesis via o-nitroanilines or the condensation of o-phenylenediamines with carboxylic acid derivatives, esters, aldehydes and aryl halides for the synthesis of benzimidazole and their derivatives.
More recently, cleaner protocols have been described, including solvent-free conditions and the use of water and ionic liquid as green solvents. However, most of these protocols use expensive and toxic reagents and/or long reaction times. Thus the development of clean, general, and selective routes to synthesize benzimidazole, including the use of new catalysts, alternative or
non-solvents, and non-classical energy sources continues to be a important field of research. Many synthetic pathways have been developed due to immense importance of benzimidazoles. The most recognize synthesis pathways involve the condensation of an arylenediamine with a carboxylic acid or its derivative under harsh dehydrating reaction conditions.
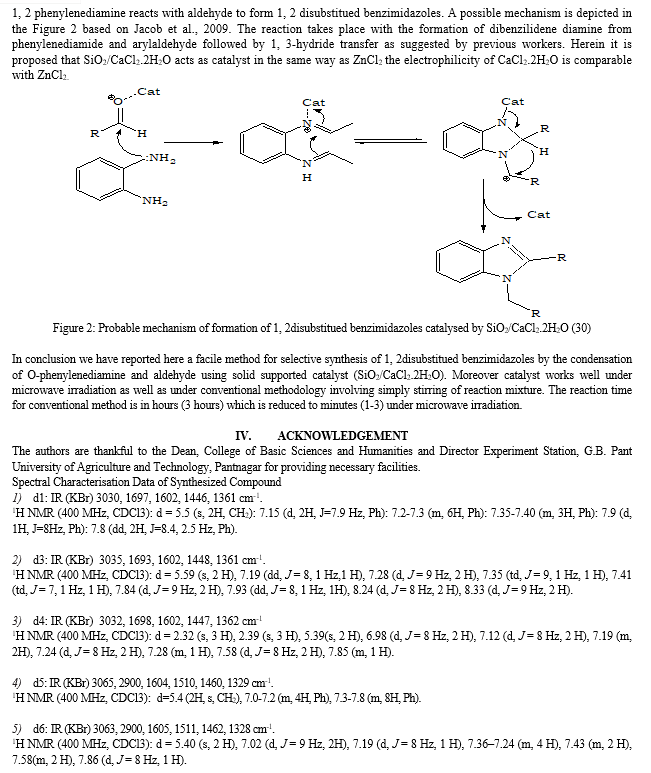
Other method is the condensation of an aldehyde with arylenediamine (17-18). According to the literature studies it reveales that several procdure for synthesis of benzimidazole and its derivatives using hypervalent iodine as oxidant (19), oxalic acid (20), H2O2/HCl (21), TiCl4 (22), PPA (23), SOCl2/SiO2 (24), L-Proline (25), Sulphamic acid (26) and Zeolite (27). Expensive reagents, oxidation processes, and long reaction times are the considerable drawbacks of these methods. In connection with our ongoing research for the development of simple and efficient methods for synthesis of benzimidazole compounds, now we report Syntheis of 1, 2 disubstitued benzimidazoles using SiO2/CaCl2.2H2O as a catalyst (28, 29)
Advantages of SiO2/CaCl2·2H2O Catalyst
- High Catalytic Activity: SiO2/CaCl2·2H2O catalyst has demonstrated excellent catalytic activity in various synthetic transformations. It promotes the reactions efficiently, resulting in high yields of desired products
- Wide Applicability: The SiO2/CaCl2·2H2O catalyst has been successfully employed in diverse organic transformations, including the synthesis of 1,2-disubstituted benzimidazoles, as well as other heterocyclic compounds and carbon-carbon bond forming reactions. Its versatility makes it a valuable tool for synthetic chemists.
- Cost-effective and Readily Available: The catalyst is composed of silica (SiO2) and calcium chloride (CaCl2·2H2O), which are inexpensive and commercially available materials. This makes the SiO2/CaCl2·2H2O catalyst a cost-effective option for both academic and industrial applications.
- Easy Separation and Recycling: SiO2/CaCl2·2H2O is a heterogeneous catalyst, which means it exists in a different phase from the reaction mixture. This property allows for easy separation by filtration or centrifugation. Moreover, the catalyst can be recovered and reused multiple times without significant loss of activity, leading to reduced waste generation and cost saving.
- Mild Reaction Conditions: The SiO2/CaCl2·2H2O catalyst operates under mild reaction conditions, typically at ambient temperature or low to moderate temperatures, and atmospheric pressure. This mildness contributes to improved safety and energy efficiency during the synthesis process.
- Environmentally Friendly: SiO2/CaCl2·2H2O is a solid catalyst, which eliminates the need for hazardous and volatile solvents often used in liquid catalyst systems. This feature enhances the environmental sustainability of the synthesis, reduces waste generation, and minimizes potential hazards associated with handling and disposal of toxic material.
- Scalability: The SiO2/CaCl2·2H2O catalyst has demonstrated its effectiveness not only in laboratory-scale reactions but also in larger-scale industrial processes. Its scalability makes it suitable for applications ranging from small-scale research to industrial production (29-31).
II. MATERIALS AND METHODS
- Formation of Solid supported Catalyst: To a 100-mL beaker, silica gel- 60 (7.5 g), CaCl2.2H2O (2.5 g), and water (3.0 mL) were added. The suspension was stirred for 15 min at room temperature, dried at 800C for 3h and for additional 15h at 150 0C in an oven and then cooled in a desicator.
- Procedure for the synthesis of 1, 2 disubstitued benzimidazoles:
a. Method A: To a mixture of aldehyde (2 mmol) and o-phenylenediamine (1 mmol) was added 0.120 g of SiO2/ CaCl2.2H2O, and the mixture was stirred at room temperature. The reaction progress was monitored over TLC. After stirring for 5.0 min to 8 hours, ethyl acetate (10 mL) was added, and the organic solution was separated from SiO2/CaCl2.2H2O by filtration. The solvent was evaporated under reduced pressure, and the residue was purified by column chromatography over silica gel eluting with hexanes:AcOEt 9:1 mixture, yielding the products.
b. Method B: SiO2/CaCl2.2H2O (0.120 g) was added to the mixture of 1, 2 phenylenediamine (1 mmol), and aldehydes (2 mmol). The mixture was placed under microwave irradiation for a period of 2-4 minutes. After the completion of the reaction (monitored over silica gel TLC) the reaction mixture was cooled to room trempture and was extracted with ethyl acetate. The solvent was evaporated in vacuo, and the residue was purified by column chromatography (silica gel) eluting with hexanes:AcOEt 9:1 mixture, yielding the products.

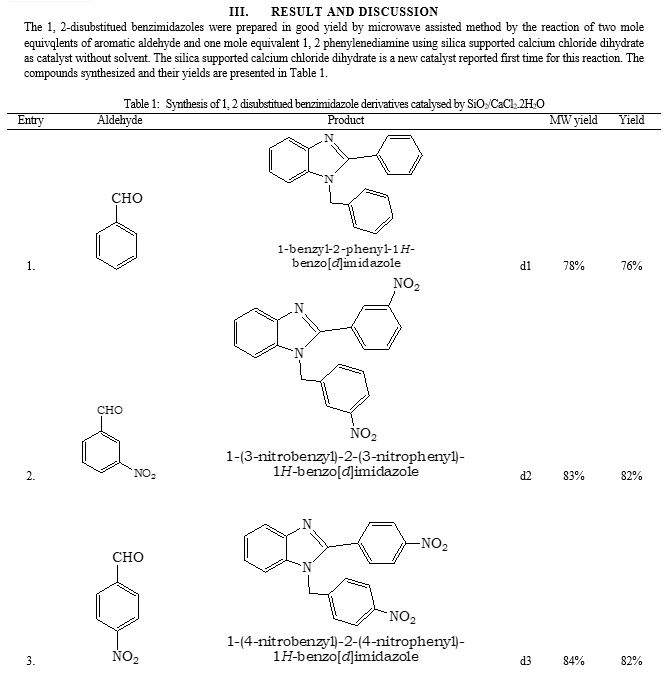
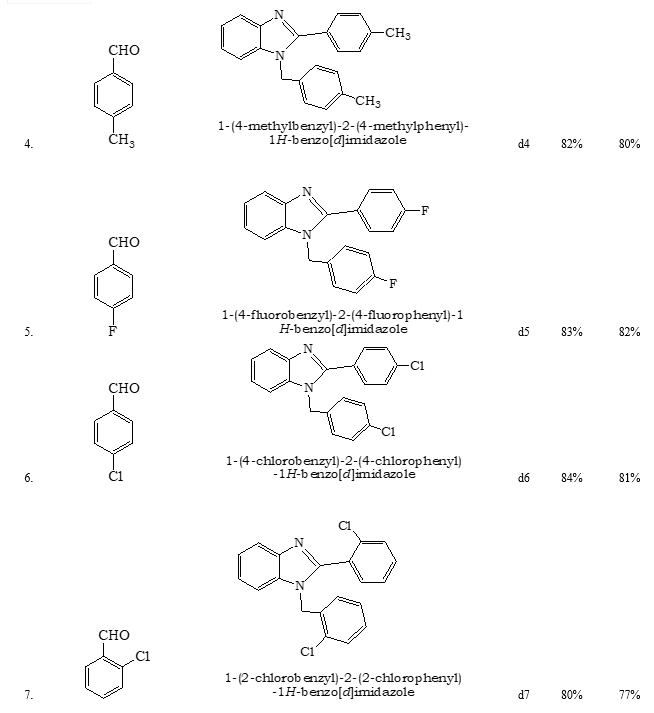
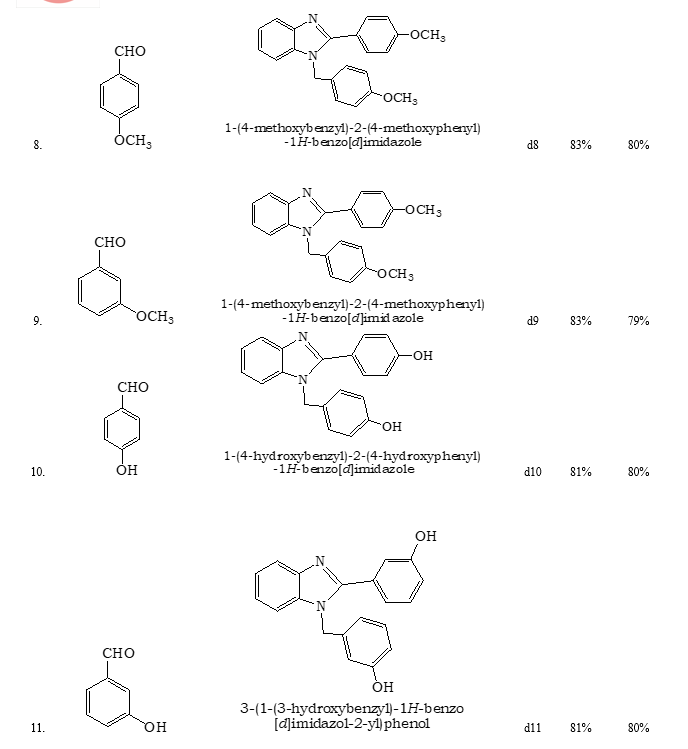
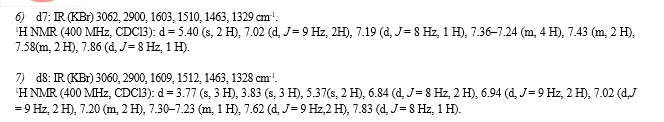
References
[1] Cano, N. H., Uranga, J. G., Nardi, M., Procopio, A., Wunderlin, D. A., & Santiago, A. N. (2016). Selective and eco-friendly procedures for the synthesis of benzimidazole derivatives. The role of the Er (OTf) 3 catalyst in the reaction selectivity. Beilstein journal of organic chemistry, 12(1), 2410-2419. [2] Erena B. & Bekdemir Y. (2014).Simple, mild, and highly efficient synthesis of 2-substituted benzimidazoles and bis-benzimidazoles and bis-benzimidazoles. Quim. Nova, 4, 643-647, [3] Patil, A., Ganguly, S., & Surana, S. (2008). A systematic review of benzimidazole derivatives as an antiulcer agent. Rasayan J. Chem, 1(3), 447-460. [4] Mohamed, B. G., Abdel-Alim, A. A. M., & Hussein, M. A. (2006). Synthesis of 1-acyl-2-alkylthio-1, 2, 4-triazolobenzimidazoles with antifungal, anti-inflammatory and analgesic effects. Acta Pharmaceutica, 56(1), 31-48. [5] Achar, K. C., Hosamani, K. M., & Seetharamareddy, H. R. (2010). In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. European journal of medicinal chemistry, 45(5), 2048-2054. [6] Khan, S. A., & Nandan, A. M. (1997). 2-Substituted benzimidazoles as antiinflammatory and analgesic agents. Indian Journal of Heterocyclic Chemistry, 7(1), 55-58. [7] KU?, C., & Altanlar, N. (2003). Synthesis of some new benzimidazole carbamate derivatives for evaluation of antifungal activity. Turkish Journal of Chemistry, 27(1), 35-40. [8] Chhonker, Y. S., Veenu, B., Hasim, S. R., Kaushik, N., Kumar, D., & Kumar, P. (2009). Synthesis and pharmacological evaluation of some new 2-phenyl benzimidazoles derivatives and their Schiff\'s bases. E-journal of chemistry, 6(S1), S342-S346. [9] Pathak, D., Siddiqui, N., Bhrigu, B., Ahsan, W., & Alam, M. S. (2010). Benzimidazoles: a new profile of biological activities. Der Pharmacia Lettre, 2(2), 27-34. [10] Theodorides, V. J., Gyurik, R. J., Kingsbury, W. D., & Parish, R. C. (1976). Anthelmintic activity of albendazole against liver flukes, tapeworms, lung and gastrointestinal roundworms. Experientia, 32(6), 702-703. [11] Demirayak, S., Kayagil, I., & Yurttas, L. (2011). Microwave supported synthesis of some novel 1, 3-Diarylpyrazino [1, 2-a] benzimidazole derivatives and investigation of their anticancer activities. European journal of medicinal chemistry, 46(1), 411-416. [12] Kumar, P. P., Dathu Reddy, Y., Ramana Reddy, C. V., Rama Devi, B., & Dubey, P. K. (2014). A New Facile Sulfenylation of 2-Acetylbenzimidazoles. Phosphorus, Sulfur, and Silicon and the Related Elements, 189(12), 1775-1779. [13] Himmel, D. M., & Arnold, E. (2020). Non-nucleoside reverse transcriptase inhibitors join forces with integrase inhibitors to combat HIV. Pharmaceuticals, 13(6), 122. [14] Walia, R., Hedaitullah, M., Naaz, S. F., Iqbal, K., & Lamba, H. S. (2011). Benzimidazole derivatives-an overview. Int. J. Res. Pharm. Chem, 1(3), 565-74. [15] Çakir, B., Yildirim, E., Ercanli, T., Erol, K., & Sahin, M. F. (1999). Synthesis and anticonvulsant activity of some (2/4-substituted) benzaldehyde (2-oxobenzothiazolin-3-yl) acetohydrazones. Il Farmaco, 54(11-12), 842-845. [16] Spasov, A. A., Yozhitsa, I. N., Bugaeva, L. I., & Anisimova, V. A. (1999). Benzimidazole derivatives: Spectrum of pharmacological activity and toxicological properties (a review). Pharmaceutical Chemistry Journal, 33(5), 232-243. [17] Soderlind, K. J., Gorodetsky, B., Singh, A. K., Bachur, N. R., Miller, G. G., & Lown, J. W. (1999). Bis-benzimidazole anticancer agents: targeting human tumour helicases. Anti-cancer drug design, 14(1), 19-36. [18] Xiangming, H., Huiqiang, M., & Yulu, W. (2007). p-TsOH catalyzed synthesis of 2-arylsubstituted benzimidazoles. Arkivoc, 13, 150-154. [19] Kim, J. S., Gatto, B., Yu, C., Liu, A., Liu, L. F., & LaVoie, E. J. (1996). Substituted 2, 5 ‘-Bi-1 H-benzimidazoles: Topoisomerase I Inhibition and Cytotoxicity. Journal of Medicinal Chemistry, 39(4), 992-998. [20] Chen, A. Y., Yu, C. H. I. A. N. G., Gatto, B., & LIu, L. F. (1993). DNA minor groove-binding ligands: a different class of mammalian DNA topoisomerase I inhibitors. Proceedings of the National Academy of Sciences, 90(17), 8131-8135. [21] Woynarowski, J. M., McHugh, M. A. R. Y., Sigmund, R. D., & Beerman, T. A. (1989). Modulation of topoisomerase II catalytic activity by DNA minor groove binding agents distamycin, Hoechst 33258, and 4\', 6-diamidine-2-phenylindole. Molecular pharmacology, 35(2), 177-182. [22] Roth, T., Morningstar, M. L., Boyer, P. L., Hughes, S. H., Buckheit, R. W., & Michejda, C. J. (1997). Synthesis and biological activity of novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 2-Aryl-substituted benzimidazoles. Journal of Medicinal Chemistry, 40(26), 4199-4207. [23] Barker, H. A., Smyth, R. D., Weissbach, H., Toohey, J. I., Ladd, J. N., & Volcani, B. E. (1960). Isolation and properties of crystalline cobamide coenzymes containing benzimidazole or 5, 6-dimethylbenzimidazole. Journal of Biological Chemistry, 235(2), 480-488. [24] Dudd, L. M., Venardou, E., Garcia-Verdugo, E., Licence, P., Blake, A. J., Wilson, C., & Poliakoff, M. (2003). Synthesis of benzimidazoles in high-temperature water. Green Chemistry, 5(2), 187-192. [25] Matloubi Moghaddam, F., Rezanejade Bardajee, G., Ismaili, H., & Maryam Dokht Taimoory, S. (2006). Facile and efficient one?pot protocol for the synthesis of benzoxazole and benzothiazole derivatives using molecular iodine as catalyst. Synthetic communications, 36(17), 2543-2548. [26] Du, L. H., & Wang, Y. G. (2007). A rapid and efficient synthesis of benzimidazoles using hypervalent iodine as oxidant. Synthesis, 2007(05), 675-678. [27] Kokare, N. D., Sangshetti, J. N., & Shinde, D. B. (2007). One-pot efficient synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles and 2, 4, 5-triaryl-1H-imidazoles using oxalic acid catalyst. Synthesis, 2007(18), 2829-2834. [28] J. P. Tripathi and V. K. Kasana (2018). Three component one pot synthesis of 1, 2-disubstituted benzimidazoles using Ticl4 as a catalyst in the microwave irraditation. International Journal of Creative Research Thoughts. (IJCRT). 6(1), 1213-1217. [29] J. P. Tripathi and V. K. Kasana (2018). Microwave Assisted Synthesis of Benzimidazoles Catalysed by Oxalic Acid International Journal for Research in Applied Science and Engineering Technology (IJRASET), 6(3),94-99. [30] Jacob, R. G., Dutra, L. G., Radatz, C. S., Mendes, S. R., Perin, G., & Lenardão, E. J. (2009). Synthesis of 1, 2-disubstitued benzimidazoles using SiO2/ZnCl2. Tetrahedron Letters, 50(13), 1495-1497. [31] Mahesha, C. K., Borade, S. A., Tank, D., Bajaj, K., Bhambri, H., Mandal, S. K., & Sakhuja, R. (2023). Tandem Transformation of Indazolones to Quinazolinones through Pd-Catalyzed Carbene Insertion into an N–N Bond. The Journal of Organic Chemistry. [32] Das, S., & Chanda, K. (2023). One-Pot Telescopic Approach to Synthesize Disubstituted Benzimidazoles in Deep Eutectic Solvent. Synthesis.
Copyright
Copyright © 2023 Jyoti Pandey Tripathi, Virendra Kumar Kasanaa. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET54392
Publish Date : 2023-06-25
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

