Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Synthesis of Novel Oxo Pyrimido Pyrimidine and Their Derivatives
Authors: Madhav S. Jadhav, Anilkumar G. Jadhav, Prashant S. Kale, Shivraj B. Sirsat
DOI Link: https://doi.org/10.22214/ijraset.2022.46125
Certificate: View Certificate
Abstract
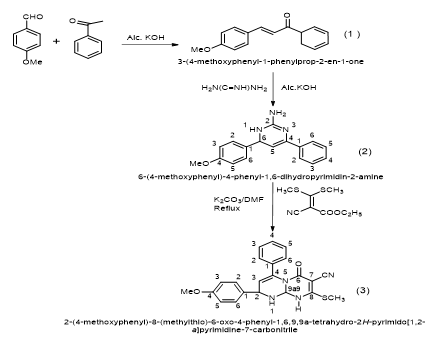
Pyrimidine derivatives are synthesized to possess various pharmacological and biological activities. In the present work, we have synthesized 3-(4-methoxyphenyl)-1-phenyl prop-2-en-1-one (1) (chalcone) by using 4-methoxybenzaldehyde and acetophenone in the presence of alcoholic KOH by Claisen-Schmidt condensation reaction. After purification and characterization by physical and spectral methods, The synthesized chalcone have been converted into 6-(4-methoxyphenyl)-4-phenyl-1,6-dihydropyrimidin-2-amine (2) by treating with guanidine nitrate in the presence of alcoholic KOH. The structure (2) has been characterized by spectral analysis. The synthesized compound (2) is further reacted with ethyl 2-cyano-3, 3-bis (methylthio)acrylate in the presence of catalytic amount of potassium carbonate in DMF under reflux condition to givenovel 2-(4-methoxyphenyl)-8-(methylthio)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a] pyrimidine-7-carbonitrile(3) in good yields. The compound (3) possesses replaceable methylthio (-SCH3) group at 8 position. The compound (3) is further reacted with various nucleophiles like substituted aromatic amines, aromatic phenols, hetryl amines and active methylene compounds to give 2-(4-methoxyphenyl)-8-substituted-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile in good yields.
Introduction
I. INTRODUCTION
The pyrimidine nucleus is present in a broad range of bioactive natural products. In addition, the pharmacological and biological activities of pyrimidine derivatives are well documented [1-2],such as antiviral, antibacterial, antitumor, anti-inflammatory, antifungal, and anti-leishmanial agents[3].Several documents have been reported for the preparation of these fused heterocycles, derivatives of which are useful as bronchodilators [4],vasodilators [5],anti-allergic [6],anti-hypertensive [7] and anti-cancer agents[4]. The presence of a pyrimidine system In thymine, cytosine, and uracil, pyrimidine system is present which are the essential building blocks of nucleic acids and one possible reason for the activity [8]. Fused derivatives of pyrimidine with thiazole are bioactive. These derivatives are used as a potent and selective inhibitors of acetyl-coA carboxylase 2[9] and VEGF receptors I and II [10]. They have also shown potent and selective human adenosine A 3 receptor [11, 12] and vanilloid receptor I TRPVI antagonism [13]. Now a days, one pot multi component reactions are gaining more importance because of environmentally implication [14].MCRs are economically and environmentally very advantageous because multistep syntheses produce considerable amounts of waste mainly due to complex isolation procedures often involving expensive, toxic and hazardous solvents after each step. Due to these advantages these reactions are perfectly suited for combinatorial library synthesis, and thus are finding increasing use in the discovery process for new drugs and agrochemicals [15-16]. Most of the MCRs are based on condensation of carbonyl group [17]. The Biginelli reaction has been employed for the synthesis of pyrimido [4, 5-d] pyrimidine [18] by the one pot condensation of acetophenone, urea and aldehydes. And most of the methods involve modification of the Biginelli reaction by condensation of aldehydes, urea and alkyl aryl ketones in acetic acid using catalytic amounts of KHSO4 [19]. Although Biginelli reaction is often employed for the synthesis of pyrimido [4, 5-d] pyrimidine by the use of base [20] and microwave assisted [21] synthesis. The other promising methods for the synthesis of pyrimido [4, 5-d] pyrimidines involve multistep syntheses starting from 1, 3-dsubstituted cyanouracils [22] polymer bound amino pyrimidine derivatives [23], aza-Wittig-type reaction [24] and reacting aminouracils with various heterocumulenes [25]. Synthetic alternatives are many, varied and have resorted to harsh conditions, example the use of PTSA (p-toluene sulphonic acid) as a catalyst, using POCl3with DMF as a solvent [26]. Additionally, reagents for these procedures are not readily or commercially available. Considering all above facts and important of pyrimido [4,5-d] pyrimidine derivatives, it is considered worthwhile to find out new methodology for synthesizing these compounds utilizing green chemistry protocol like using eco-friendly reagents and catalysts, solvent free or reaction in non-hazardous solvent, because it offers enhanced chemical process economics concomitant with a reduced environmental burden.The structures of the various synthesized compounds were assigned on the basis of IR, 1H NMR, 13C NMR and Mass spectral data.
In the view of this observation and extension of earlier work, we have synthesized 2-(4-methoxyphenyl)-8-(methylthio)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a] pyrimidine-7-carbonitrile (3) by using6-(4-methoxyphenyl)-4-phenyl-1,6-dihydropyrimidin-2-amine (2) [27-28 ] and ethyl 2-cyano-3, 3-bis(methylthio)acrylate. 6-(4-methoxyphenyl)-4-phenyl-1,6-dihydropyrimidin-2-amine (2) is prepared by the reaction of 3-(4-methoxyphenyl)-1-phenyl prop-2-en-1-one (1)chalcone [29-30] with guanidine nitrate in the presence of alcoholic potassium hydroxide under reflux condition.
II. METHODS
Melting points were determined in open capillary tubes and are uncorrected. The silica gel F254 plates were used for thin layer chromatography (TLC); the spots were examined under UV light and then developed in an iodine vapor. Column chromatography was performed with silica gel (BDH 100-200 mesh). Solvents were purified according to standard procedures. The spectra were recorded as follows: IR, KBr pellets, a Perkin-Elmer RX1 FT-IR spectrophotometer; 1H NMR, CDCl3, 200 MHz, a Varian Gemini 200 instrument. Elemental analysis was performed on a Heraeus CHN-O rapid analyzer.
A. 2-(4-methoxyphenyl)-8-(methylthio)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile..
- Step – I: A solution of KOH 50% is added to an equimolar solution of acetophenone (0.01mole) and 4-methoxybenzaldehyde (0.01 mole) in ethanol 95%; the addition is performed under energetic stirring at room temperature. The reaction is left under stirring for one night and then diluted with water and acidified; the precipitate is separated by filtration and dried under vacuum. They are crystallized by ethanol compound.
- Step – II: A mixture of chalcone i.e. 3-(4-methoxyphenyl)-1-phenylprop-2-en-1-one (2.38 gm,0.01mole ), and guanidine nitrate (1.20 g 0.01 mole ) were dissolved in alcoholic potassium hydroxide solution (10 ml ) was heated for 4 hrs., then it was poured into cold ice obtained 6-(4-methoxyphenyl)-4-phenyl-1,6-dihydropyrimidin-2-amine.
- Step – III: A mixture of 6-(4-methoxyphenyl)-4-phenyl-1,6-dihydropyrimidin-2-amine (2) and ethyl 2-cyano-3,3-bis(methylthio)acrylate in the presence of catalytic amount of potassium carbonate (10 mg) in DMF was refluxed for 4 hours, the reaction was monitored by TLC. After completion, the reaction mixture was cooled at room temperature then wash with water and extracted with ethyl acetate. The extract was concentrated and the residue was subjected to column chromatography (silica gel, n-hexane-ethyl acetate 8:2) to obtain pure solid compound 2-(4-methoxyphenyl)-8-(methylthio)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile (3). The compound (3) is confirmed by IR, 1H NMR, C13 NMR and MS analytical data.
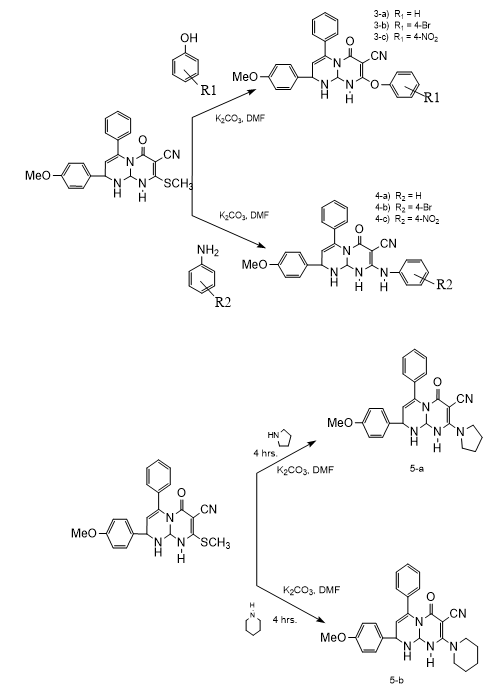
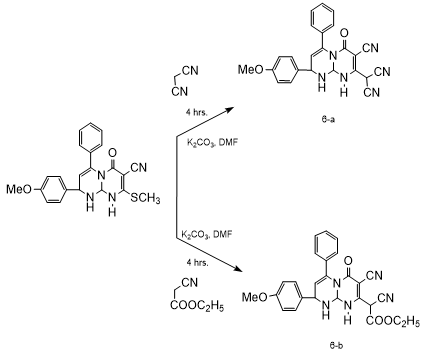
B. Synthesis of Derivatives
A mixture of compound (3) (1mmol) and independently, various substituted aromatic amines, aromatic phenols, hetryl amines and active methylene compounds (1mmol) in DMF (10 ml) and anhydrous potassium carbonate (10 mg ) was reflux for 4 to 6 hrs. The reaction mixture cooled to room temperature and poured into ice cold water. The separated solid product was filtered, washed with water and recrystllized by using ethyl alcohol.
III. RESULT AND DISCUSSION
The compound 2-(4-methoxyphenyl)-8-(methylthio)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile are synthesized by dissolving 6-(4-methoxyphenyl)-4-phenyl-1,6-dihydropyrimidin-2-amine (2) and ethyl 2-cyano-3-3-bis(methylthio)acrylate in the presence of K2CO3 in DMF under reflux condition. The synthesized compound acts as electrophilic species reacting with various substituted aromatic amines, aromatic phenols, hetryl amines and active methylene compound gives 2-(4-methoxyphenyl)-8-(methylthio)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile in good yields.
IV. ACKNOWLEDGEMENTS
The authors are grateful to Dr. G.N. Shinde, Principal, Yeshwant Mahavidyalaya, Nanded for providing laboratory facilities and Vishnu Chemical Ltd, Hyderabad, for providing spectral data.
Conclusion
A new different 2-(4-methoxyphenyl)-8-(methylthio)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrileare synthesized by using simple and efficient chemistry and this synthesized compounds possesses methylthio group at 8-position which is best leaving group therefore synthesized compound (3) act as an electrophilic species and it reacts with various nucleophiles. 3) 2-(4-methoxyphenyl)-8-(methylthio)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile. IR : 3360, 2260,1715, 1630, 1030 cm-1; 1HNMR : ?2.38(s,3H,SCH3),5.10(s,1H,NH),4.92(s,1H,NH),5.86(s,1H,=CH),4.51(s,CH),5.82(s,1H,CH),7.65(s,5H,Ar-H),7.12(dd,2H,Ar-H),6.69(dd,2H,Ar-H),3.79(s,3H,OCH3). ESI-MS : 404. Anal.Calcd for C22H20N4O2S: C, 65.33; H,4.98; N,13.85;O,7.91;S, 7.93 Found : C,62.63;H,4.52;N, 9.96;O,7.63;S,15.26. Mol. Formula: C22H20N4O2S Mol.Wt. : 404. 3-a) 2-(4-methoxyphenyl)-6-0xo-8-phenoxy-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile. IR : 3390, 2220, 1700, 1620, 1050, cm-1; 1H NMR : 5.10(s,1H,NH), 6.47 (s,1H,=CH) 5.75 (s,1H,CH), 5.52 (s,1H,CH),7.24 (s,5H,Ar-H),6.90(dd,2H,Ar-H),7.21 (dd,2H,Ar-H),7.05(s,5H,Ar-H),3.80(s,3H,OCH3) ESI-MS : 450. Anal. Calcd. : C27H22N4O3C,71.99;H,4.92;N,12.44;O,10.65 Found : C, 69.34; H, 4.52; N, 8.98; O, 10.28; S, 6.88. Mol. Formula: C27H22N4O3 Mol.Wt. : 450. 3-b) 8-(4-bromophenoxy)-2-(4-methoxyphenyl)-6-oxo-4-phenyl-1,6,9,9a-tetra-hydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile. IR : 3370, 2260, 1705, 1640, 1020,670 cm-1; 1H NMR : 5.11(s,1H,NH), 6.46 (s,1H,=CH) 5.73 (s,1H,CH), 5.54 (s,1H,CH),7.19(s,5H,Ar-H),6.94 (dd,2H,Ar-H),7.23 (dd,2H,Ar-H),6.88 (dd,2H,Ar-H),7.24 (dd,2H,Ar-H),3.78 (s,3H,OCH3) ESI-MS : 529. Anal. Calcd : C27H21BrN4O3:C,61.26; H,4.00; Br,15.09; N,10.58; O,9.07 Found : C,59.31; H, 3.62; Br,14.60; N,7.71; O,8.83; S,5.93. Mol. Formula: C27H21BrN4O3 Mol.Wt : 529. 3-c) 2-(4-methoxyphenyl)-8-(4-nitrophenoxy)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile. IR : 3365, 2275, 1710, 1650, 1070,1570 cm-1; 1H NMR : 5.09(s,1H,NH), 6.49 (s,1H,=CH) 5.76 (s,1H,CH), 5.53 (s,1H,CH), 7.22(s,5H,Ar-H),6.92 (dd,2H,Ar-H),7.23 (dd,2H,Ar-H), 7.18 (dd,2H,Ar-H),7.96(dd,2H,Ar-H), 3.76 (s,3H,OCH3) ESI-MS : 495. Anal. Calcd : C27H21N5O5: C,65.45; H,4.27; N,14.13; O,16.14 Found : C,63.29; H,3.91; N,10.91; O,15.62, S,6.27. Mol. Formula : C27H21N5O5 Mol.Wt. : 495. 4-a) 2-(4-Methoxyphenyl)-6-oxo-4-phenyl-8-(phenylamino)-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile. IR : 3365, 2285, 1700, 1650, 1050, cm-1; 1H NMR : 5.10(s,1H,NH),4.10(s,1NH), 6.43 (s,1H,=CH) 5.75 (s,1H,CH), 5.54 (s,1H,CH), 7.20(s,5H,Ar-H),6.89(dd,2H,Ar-H),7.25(dd,2H,Ar-H),7.01(s,5H,Ar-H),3.80 (s,3H,OCH3). ESI-MS : 449. Anal. Calcd : C27H23N5O2: C,72.14; H,5.16; N,15.58; O,7.12 Found : C, 69.48; H,4.81;N,12.03; O,6.83;S,6.85 Mol. Formula: C27H23N5O2 Mol.Wt. : 449. 4-b) 8-((4-bromophenyl)amino)-2-(4-methoxyphenyl)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile. IR : 3320, 2260, 1710, 1630, 1050,660 cm-1; 1H NMR : 5.08(s,1H,NH),4.13(s,1NH), 6.46 (s,1H,=CH) 5.76 (s,1H,CH),5.50 (s,1H,CH),7.22(s,5H,Ar-H),6.92(dd,2H,Ar-H),7.23(dd,2H,Ar-H),6.49(dd,2H,Ar-H),7.32 (dd,2H,Ar-H),3.78(s,3H,OCH3) ESI-MS : 528 Anal. Calcd : C27H22BrN5O2: C, 61.37; H,4.20;Br,15.12;N, 13.25; O, 6.06 Found : C,59.42; H,3.91; Br,14.58; N,10.32; O,5.89; S,5.88 Mol. Formula : C27H22BrN5O2 Mol.Wt. : 528. 4-c) 2-(4-methoxyphenyl)-8-((4-nitrophenyl)amino)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile. IR : 3370, 2280, 1705, 1670, 1050, cm-1; 1H NMR : 5.11(s,1H,NH),4.09(s,1NH), 6.44 (s,1H,=CH) 5.72 (s,1H,CH), 5.56 (s,1H,CH),7.21(s,5H,Ar-H),6.88(dd,2H,Ar-H),7.25 (dd,2H,Ar-H),6.63 (dd,2H,Ar-H),7.98 (dd, 2H,Ar-H), 3.80(s,3H,OCH3) ESI-MS : 494. Anal. Calcd : C27H22N6O4: C,65.58; H,4.48; N,16.99; O,12.94 Found : C,63.45; H,4.13; N,13.67; O,12.48; S,6.27. Mol. Formula : C27H22N6O4 Mol.Wt. : 494. 5-a) 2-(4-methoxyphenyl)-6-oxo-4-phenyl-8-(pyrrolidin-1-yl)-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile. IR : 3380, 2265, 1715, 1650, 1050, cm-1; 1H NMR : 5.10 (s,1H,NH), 6.45 (s,1H,=CH) 5.76 (s,1H,CH), 5.52 (s,1H,CH),7.22 (s,5H,Ar-H),6.90(dd,2H,Ar-H),7.23 (dd,2H,Ar-H),3.81 (s,3H,OCH3),2.60 (t,4H),1.65(m,4H) ESI-MS : 427. Anal. Calcd : C25H25N5O2, C,70.24; H,5.89; N,16.38; O,7.48 Found : C,67.57; H,5.41; N, 12.56; O,7.23; S,7.23 Mol. Formula : C25H25N5O2 Mol.Wt. : 427 5-b) 2-(4-methoxyphenyl)-6-oxo-4-phenyl-8-(piperidin-1-yl)-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidine-7-carbonitrile. IR : 3360, 2240, 1715, 1630, 1070, cm-1; 1H NMR : 5.13(s,1H,NH), 6.48(s,1H,=CH) 5.72 (s,1H,CH), 5.54 (s,1H,CH),7.20 (s,5H,Ar-H),6.86 (dd,2H,Ar-H),7.23(dd,2H,Ar-H),3.77 (s,3H,OCH3), 3.13(t,4H),1.53 (m,6H) ESI-MS : 441. Anal. Calcd : C26H27N5O2, C,70.73; H,6.16; N,15.86; O,7.25 Found : C,68.18; H, 5.69; N,12.22; O,6.94; S,6.97. Mol. Formula : C26H27N5O2 Mol.Wt. : 441. 6-a) 2-(7-cyano-2-(4-methoxyphenyl)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidin-8-yl)malononitrile. IR : 3335, 2970, 2270,1720, 1640, 1030 cm-1; 1H NMR : 5.10(s,1H,NH), 6.49 (s,1H,=CH) 5.73 (s,1H,CH), 5.51 (s,1H,CH),7.23 (s,5H,Ar-H),6.91 (dd,2H,Ar-H),7.22 (dd,2H,Ar-H), 4.16 (s,1H,act.-CH),3.81 (s,3H,OCH3) ESI-MS : 422. Anal. Calcd : C24H18N6O2,C,68.24; H,4.29; N,19.89; O,7.57 Found : C,65.55; H,3.92; N,15.92; O,7.30; S,7.31. Mol. Formula : C24H18N6O2 Mol.Wt. : 422. 6-b) ethyl 2-cyano-2-(7-cyano-2-(4-methoxyphenyl)-6-oxo-4-phenyl-1,6,9,9a-tetrahydro-2H-pyrimido[1,2-a]pyrimidin-8-yl)acetate. IR : 3355, 2265, 1715, 1625, 1050,2910,1760cm-1; 1H NMR : 5.08(s,1H,NH), 6.44 (s,1H,=CH), 5.76 (s,1H,CH), 5.51 (s,1H,CH),7.27 (s,5H,Ar-H),6.89(dd,2H,Ar-H),7.22(dd,2H,Ar-H), 3.98 (s,1H,act.-CH),3.79 (s,3H,OCH3), 4.19 (q,2H), 1.22 (t,3H). ESI-MS : 469. Anal. Calcd : C26H23N5O4: C,66.51; H,4.94; N,14.92; O,13.63 Found : C, 65.74; H,4.44; N,12.28; O,10.53; S,7.01. Mol. Formula : C26H23N5O4 Mol.Wt. : 469.
References
[1] H.Numazi, Y.R. Marlena, H. Azamat, Heterocycl. Chem., 2001,38,1051. [2] C.O.Kappe, Eur.J.Med Chem., 2000,35,1043. [3] (a) M.B.Deshmukh, S.M. Salunkhe,D. R. Patil, P.V. Anbhule, Eur.J.Med.Chem., 2009, 44, 2651; (b) M. J. Aliaga, D.J. Ramon, M. Yus, Org. Biomol.Chem., 2010,8,43. [4] W. J. Coates, European Patent 351058, 1990; Chem. Abstr. 1990, 113, 40711 [5] J.D. Figueroa-Villar, C. L. Carneiro, E. R. Cruz, Heterocycles, 1992, 34,891. [6] N. Kitamura, A. Onishi, European Patent163599, 1984; Chem. Abstr. 1984, 104, 186439. [7] R. Gupta, A. Jain, R. Joshi, M. Jain, Bull. Korean Chem. Soc., 2011, 32,899. [8] A. Amir, s. A. Javed. Kumar, Indian. J. Pharm. Sci., 2007, 69, 337. [9] F.R.Clark, T.Zhang,X. Wang,R.Wang, X. Zhang,S.H.Camp, B.A. Beutel, H. L. Sham, G. Y. Gu, Med. Chem. Lett., 2007,17,1961. [10] A. S. Kiselyov, E. Piatanitski, M. Semenova, V. V. Semenov, Bioorg. Med. Chem. Lett. 2006, 16, 602. [11] Y. K. Jung, K. S. Kim, G. Z. Geo, a. S. Gross, N. Melman, K. A. Jacobson, C. Y. Kim, Bioorg. Med.Chem. 2004, 12,613. [12] P. Bhattacharya, T.J. Leonard, K.Roy, Bioorgan. Med. Chem., 2005,13,1159. [13] N, Xi, Y, Bo,E, M. Doherty, C. Fotsch, N. R. Gawa, N. Han, R. W. Hungate, L. Kolinsky, Q. Liu, R. Tamir, Bioorg. Med. Chem. Lett., 2005,15,5211. [14] C. C. A. Cariou, G. J. Clarkson, Shipman, J. Org. Chem., 2008,73,9762. [15] A. Domling, I. Ugi, Angew. Chem. Int. Ed., 2000, 39, 3168. [16] (a) I. Ugi, A. Domling, endeavor 1994, 18, 115; (b) S. Heck, A. Domling, Synlett 2000,424. [17] A. Bazgira, M. M. Khanaposhtani, A. A.Soorki, Bioorg. Med. Chem. Lett., 2008, 18, 5800. [18] V. F. Sedona, O. P. Shkurko, chem. Heterocycl. Compd. (Engle Transl) 2004, 40,194; (b) R. L. Magar, P.B. Throat, P. B. Throat, V. V. Throat, B. R. Patil, R. P. Pawar, Chin. Chem.Lett. 2013,24,1070. [19] F. Shi, R. Jia, X. Zhang, S.Tu, S.Yan, Y.Zhang. B. Jiang, J. Zhang, C. Yao, Synthesis, 2007, 2782. [20] N. Sharm, V. Rane, K. Gurram, Bioorg. Med.Chem. Lett., 2004, 14, 4185. [21] H. Dabiri, H. Arvin-Nezhad,R. Khavasi, A. Bazgir, J. Heterocyclic Chem., 2007,44,1009. [22] K. Hirota, Y. Kitade, H. Sajiki, Y. Maki, J. Chem. Soc., Perkin Trans.,1 1990,123. [23] S. K. Srivastava, W. Haq, P. M. S. Chauhan, Bioorg. Med. Chem. Lett., 1999, 9,965. [24] H. Wamhoff, J. Muhr, Synthesis, 1998,919. [25] D. Prajapati, A.J. Thakur, Tetrahedron Lett., 2005,46,1433. [26] K. Hirota, Y. Kitade, H. Sajiki, Y. Maki, Synthesis, 1984,589. [27] R.Kushal Lanjewar, S. Mukund and D.Binda . Synthesis and antimicrobial activity of 5-(2-aminothia-zol-4-yl)-3,4-dihydro-4-phenyl Pyrimidin-2(1H)-one: Synthesis of pyrimidine compound Indian J. Chem.2009,48.1732 [28] A, Manjula, B, Rao and P, Neelakantam. An inexpensive protocol for Biginelli reaction: Synthesis of pyrimidine compound Synth. Commun.2004,34.2665. [29] YR, Prasad. AL,Rao. And R, Rambabu. Synthesis and Antimicrobial activity of some chalcone Derivatives.E-Journal of Chemistry. 2008,5(3):461-466. [30] Sj, Won. CT, Liu. LT, Tsao. HH,Ko. JP,Wang. CN, Lin. Synthetic Chalcones as potential anti-inflammatory and cancer chemo protective agents. European Journal of Medicinal Chemistry.2005,40.103-112.
Copyright
Copyright © 2022 Madhav S. Jadhav, Anilkumar G. Jadhav, Prashant S. Kale, Shivraj B. Sirsat. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET46125
Publish Date : 2022-08-02
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

