Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Bacteriological Assessment of Lake Water samples in Raipur, Chhattisgarh: A Comprehensive Analysis of Water Samples
Authors: Dr. Pooja Narwat
DOI Link: https://doi.org/10.22214/ijraset.2024.61110
Certificate: View Certificate
Abstract
The assessment of water quality is crucial, relying on both physico-chemical and microbiological standards. A comprehensive understanding of these parameters is essential to guide quality maintenance. Bacterial activity plays a pivotal role in decomposition, significantly influencing nutrient turnover and contributing to the biogeochemical cycle\'s foundation. Microbial diversity and dominance are integral to the biogeochemical cycle. However, bacterial and viral diseases transmitted through water, such as cholera, typhoid, and hepatitis, pose significant health risks during monsoon and summer seasons. Different regions adhere to various standards for water and potable water. Lakes play a vital role in determining groundwater quality, the primary source of potable water for rural populations, often consumed with minimal treatment. The quality of lake water is a matter of concern for public health, particularly due to the presence of indicator bacteria such as fecal coliforms, fecal streptococci, fecal staphylococci, and Enterobacteriaceae. In this study, lake water samples were meticulously analyzed for various bacterial loads, including total coliforms, fecal coliforms, E. coli, Staphylococcus, and Streptococcus. These bacterial indicators not only serve as determinants of water quality but also raise serious concerns about the health of humans and animals relying on these water bodies. In summary, this research delves into the microbiological aspects of water quality in lakes, emphasizing the significance of bacterial indicators and their potential impact on public health. The findings contribute valuable insights for developing strategies to safeguard water quality and mitigate health risks associated with waterborne diseases.
Introduction
I. INTRODUCTION
Water, nature's precious gift, is essential for life, supporting diverse activities such as drinking, agriculture, industry, power generation, and recreation. However, escalating demand, coupled with pollution from urbanization and industrialization, poses a global water crisis. Groundwater is depleting day by day. With limited water resources, efficient management of these resources is vital. Limnology is the study of fresh water including their physical & biological aspects.
A variety of microorganisms are found in water samples. Zooplankton are tiny organisms that drift aimlessly in water currents in aquatic ecosystems, comprising a diverse array of microscopic animals. Their abundance and diversity reflect the health and productivity of the water body, making them valuable indicators for water quality assessments. Phytoplankton are microscopic, photosynthetic organisms that are found throughout the lighted regions of aquatic environments, primarily composed of algae and cyanobacteria. Their abundance and diversity influence water clarity and nutrient cycles, making them vital components in assessing the overall ecological balance of lakes and oceans. Apart from these, bacteria is also present in lake water.
Bacteriological analysis of lake water samples involves the examination of water for the presence and concentration of bacteria. This process is crucial for assessing water quality, identifying potential health risks, and determining the overall ecological health of a lake. Common bacteria of concern in lake water include pathogenic bacteria that can cause waterborne diseases.
Key steps in bacteriological analysis of lake water samples include sample collection, transportation, and laboratory analysis. Water samples are collected at various depths and locations to ensure representative results. Once collected, samples are transported to a laboratory under controlled conditions to prevent contamination.
In the laboratory, the water samples undergo various tests to determine bacterial concentrations. These tests may include total coliforms, fecal coliforms, and Escherichia coli (E. coli) counts. Coliform bacteria serve as indicators of potential fecal contamination, while E. coli is specifically associated with fecal material and is often used as an indicator of water safety.
The results of bacteriological analysis help authorities make informed decisions about water treatment, public health advisories, and environmental management. Regular monitoring of lake water through bacteriological analysis is essential for ensuring the safety of drinking water sources, protecting public health, and maintaining the ecological balance of aquatic ecosystems.
Bacteriological analysis of lake water samples is a crucial aspect of assessing water quality and understanding potential health risks associated with recreational activities or water consumption. Lakes, being intricate ecosystems, can be susceptible to microbial contamination, posing threats to both environmental health and human well-being. This analysis involves the examination of bacterial communities present in the water, focusing on indicators such as coliform bacteria, Escherichia coli (E. coli), and other pathogenic microorganisms. The presence of these bacteria can indicate the level of fecal contamination and, consequently, the potential for waterborne diseases.
II. REVIEW OF LITERATURE
The field of limnological studies owes much of its foundation to pioneering researchers such as Waters by Bridge (1915), Thienemann (1922), Atkin and Harris (1926), Pearshall (1978), Jankin (1942), and Lindeman (1942). These early scholars not only established the fundamental principles of limnology but also provided invaluable guidelines and inspiration for contemporary researchers.
The planktonic and bacteriological study are very useful tool for the assessment of water quality in water body and also contributes to understanding of the basic nature and general economy of the lake. The maintenance of a healthy aquatic ecosystem depends on the abiotic properties of water and the biological diversity of the ecosystem.
III. MATERIALS AND METHODS
A. Sample Site
Raipur is the capital city of the Indian state of Chhattisgarh and the largest city of the state. The wetlands of New Raipur is considered as ecological important wetlands. Ecological wetlands are among the most productive ecosystems in the world. They also form a source of substantial biodiversity in supporting numerous species The wetlands selected for bacteriological analysis are Parsada lake, Bandha dam, Khuteri Lake I, Khanduwa dam, Khuteri lake II, Chherkapur lake, Maharajabandh lake.
B. Sample Collection
Water samples were systematically collected for bacteriological analyses throughout different seasons from October to July. These samples were gathered in pre-sterilized, dark-colored plastic cans to prevent external contamination. Collection was conducted away from the shore at a depth of 1.5 feet. Samples were preserved by adding few drops of lugol’s iodine.
IV. BACTERIOLOGICAL ANALYSIS
- Enumeration of aerobic bacteria by aerobic count method (APC)
Serially diluted lake water sample was inoculated on plate count agar media by pour Plate technique in duplicates and the plates were incubated at 37°C for 24 h. Total number of colonies developed were counted with the help of colony counter and colony forming unit (CFU) ml-1 was calculated.
2. Enumeration and identification of bacteria by heterotrophic plate count
were incubated at 37°C for 24 h. CFU ml-1 was calculated and the isolates were pure cultured on nutrient agar slants and the colony characteristics were identified. The cells were subjected to gram staining and biochemical test for identification.
3. Enumeration of MPN total coliforms (TC) Presumptive test
Ten milliliters of a water sample were inoculated into five tubes of double-strength Lauryl Sulphate Tryptose broth (LST broth) with Durham’s tube. Additionally, 1 ml and 0.1 ml of the inoculum were added to 10 tubes of single-strength LST broth. Phenol red was used as pH indicator. The tubes indicating acid production by changing the color from red to yellow. After incubation at 37°C for 24 hours, positive tubes indicating acid and gas production were confirmed, and MPN 100 ml -1 was calculated.
4. Confirmatory test
Inoculum from positive tubes were transferred to Brilliant green lactose bile (BGLB) broth tubes containing Durham’s tubes and were incubated at 370 C. Formation of gas indicate positive test.
The inoculum from positive tube was transferred on EMB agar plate by streak plate method and was incubated at 37 0 C for 48 h for E.coli confirmation. The plates were observed for metallic green sheen colonies.
5. Completed test
The colonies having metallic green sheen were pure cultured on nutrient agar slants, incubated at 370 C for biochemical test.
6.. Enumeration of MPN fecal coliforms (FC)
The positive presumptive test tube for total coliforms was inoculated into E. coli broth with Durham’s tube and incubated at 44.5°C for 24 hours. Gas production indicated a positive result, and MPN for fecal coliforms was calculated. Confirmation involved transferring the positive tube to BGLB broth, observing gas production, and inoculating EMB and MacConkey’s agar for E. coli and other coliform group confirmation, respectively.
7. Enumeration of MPN fecal streptococci
Single and double strength Azide Dextrose broth tubes were inoculated with 10 ml, 1 ml, and 0.1 ml of lake water sample. Incubation was done at 37°C, positive tubes were counted for MPN calculation. Positive Azide Dextrose broth was streaked on Pfizer Selective Enterococci Agar (PSE) and incubated at 37°C. Confirmation of fecal streptococci was done by observing brownish-black colonies with brown halos. Confirmed colonies underwent gram staining and biochemical tests.
8. Enumeration of MPN Staphylococci
Single and double strength Baird Staphylococcus Enrichment Broth (BSE broth) tubes were inoculated with 10 ml, 1 ml, and 0.1 ml of lake water sample. After incubation at 37°C, a positive culture was streaked onto Baird-Parker (BPA) agar. Isolated staphylococci were maintained on agar slant and subjected to gram staining and biochemical tests.
9. Escherichia coli count by membrane filtration
About 100 ml lake water sample was filtered through 0.45 µm membrane filters. Filter discs were placed on EMB agar and incubated at 37°C. E. coli colonies were counted, and biochemical tests were conducted for confirmation.
10. p/a test using H2S strip
The identification of H2S-producing pathogenic bacteria involved filling an H2S strip bottle to the mark with lake water sample and incubating it for 24-48 hours at 34°C. The cultured sample on agar plates underwent further identification through the triple sugar iron test, enabling the characterization of colonies.
11. Identification of bacterial isolates
Preliminary colonies from selective media were differentiated based on morphological features and transferred to nutrient broth, incubated at 37°C. Pure isolates, identified through colony morphology and Gram staining, were preserved. Identification followed Bergey's manual (2000) protocols, with biochemical test kits confirming isolates.
12. Turkey’s test
Variations in bacteriological parameters were compared using one-way ANOVA. For statistically significant results, Turkey's post hoc test for multiple comparisons was conducted. Seasonal variations were analyzed using the paired t-test.
Enumeration of aerobic bacteria by aerobic plate count (APC) and heterotrophic plate count method (HPC)


V. RESULT AND DISCUSSION
Water quality assessment hinges on microbiological standards crucial for maintaining and guiding quality. Bacterial activity, particularly decomposition, plays a pivotal role in nutrient turnover. Microbial diversity forms the foundation of biogeochemical cycles. Waterborne diseases like cholera, typhoid, and hepatitis pose threats, especially during monsoon and summer seasons. Varying regional standards exist for water quality and potable water. WHO recommends 10 MPN 100 ml-1 for coliforms and zero for fecal coliforms.
Lakes impact groundwater quality, serving as a rural potable water source, often consumed with minimal treatment. Indicator bacteria like fecal coliforms, fecal streptococci, fecal staphylococci, and Enterobacteriaceae raise public health concerns. Lake water analysis for total coliforms, fecal coliforms, E. coli, Staphylococcus, and Streptococcus is crucial for water quality and the well-being of humans and animals dependent on these water sources.
Tables 9.1-9.7: Seasonal variations of bacteriological parameters for the study period
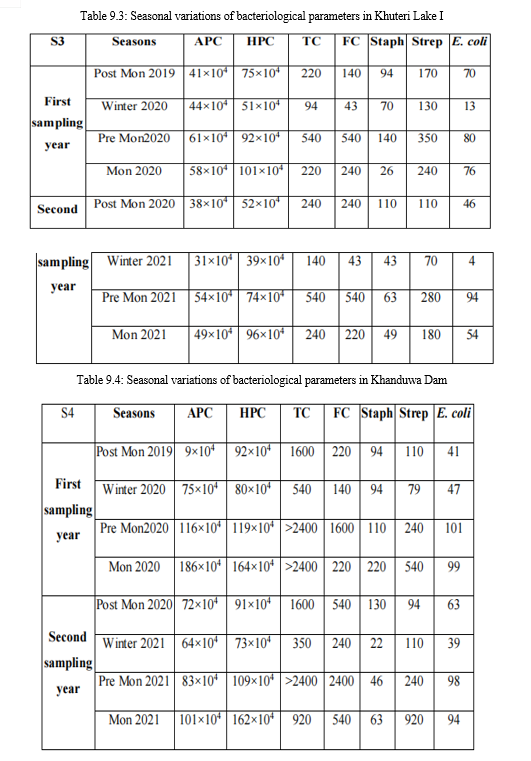
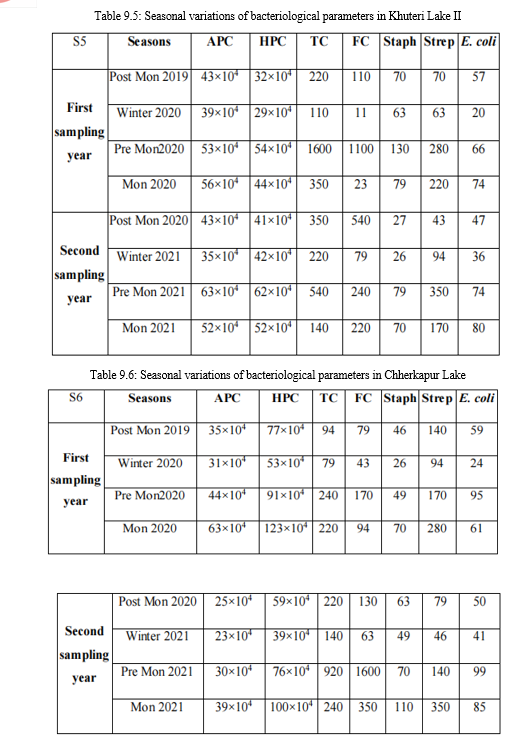
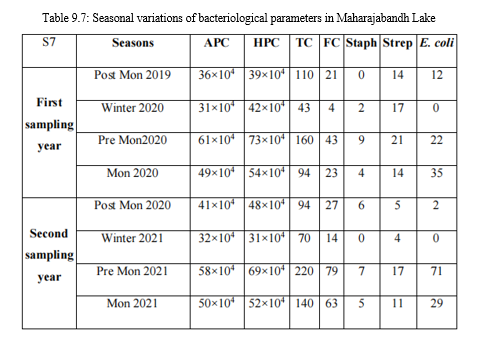
APC: Aerobic Plate Count (CFU ml-1 ); HPC: Heterotrophic Plate Count (CFU ml-1 ); TC: Total Coliform (MPN 100 ml-1 ); FC: Fecal Coliform (MPN 100 ml-1 ); Staph: 106 fecal staphylococci (MPN 100 ml-1 ); Strep: fecal streptococci (MPN 100 ml-1 ); E. coli: Escherichia coli (CFU 100 ml-1 )
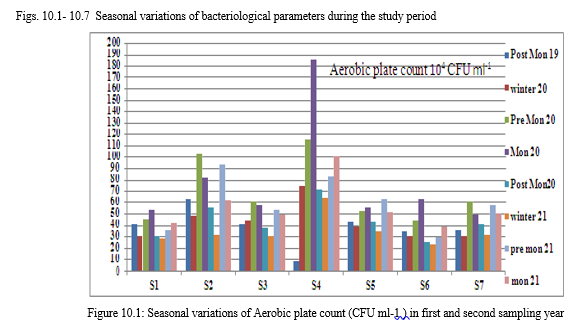
A. Aerobic plate count
The overall APC range during the sampling period was 186×104 CFU ml-1 to 9×104
CFU ml-1. APC count was comparatively high in S4 followed by S2 and S5. The range was usually high in monsoon and pre monsoon seasons, which indicate the direct effect of human activities on bacterial population. The high temperature and the entry of certain particles in to the lake along with rain water runoff may positively affect the increase the growth of bacteria. The count was low in winter and the low temperature and low organic input plays an important in this variation.
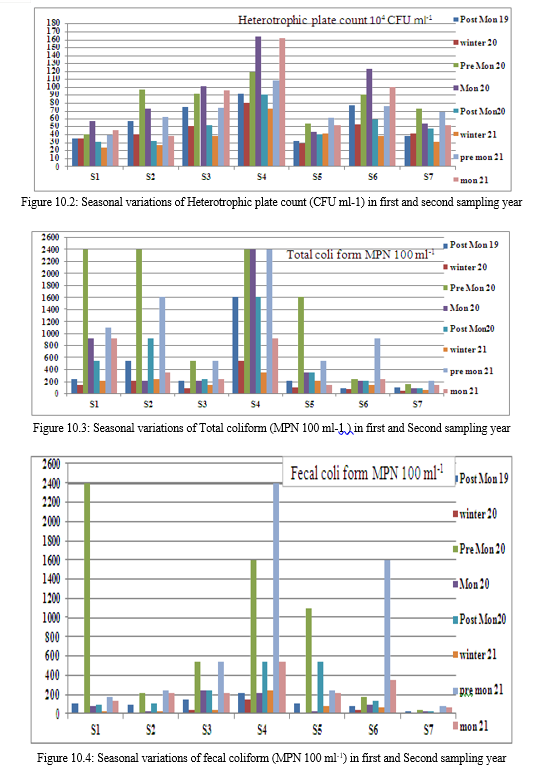
B. Heterotrophic plate count
The Heterotrophic Plate Count (HPC), a primary bacterial parameter endorsed by the Environmental Protection Agency (EPA), offers a quick, reliable, and cost-effective measure for assessing water quality. During the sampling period, HPC ranged from 164×104 to 24×104 CFU ml-1, peaking in monsoon and pre-monsoon seasons due to favorable temperature and nutrient-rich rainwater runoff. Winter saw lower counts due to decreased temperature. Sample S4 consistently exhibited higher counts, followed by S6 and S3. Heterotrophic activity fluctuated throughout the year, crucial in decomposition processes. Elevated HPC suggests microbial nutrient richness, posing a potential risk of eutrophication in the lake water.
C. Total coliform
The total coliform count ranged from 2400 MPN 100 ml-1 to 43 MPN 100 ml-1 during the sampling period. Sample S4 consistently had the highest counts, followed by S1, while S2 and S5 showed lower counts. Pre-monsoon exhibited the highest counts, followed by monsoon and post-monsoon, with low counts in winter due to lower temperatures. According to the Central Pollution Control Board (CPCB), the water falls under class 'C,' requiring conventional disinfection.
D. Fecal coliform count
Total fecal coliform ranged from 2400 MPN 100 ml-1 to 4 MPN 100 ml-1, peaking in pre-monsoon and decreasing in winter. S4 had the highest counts, followed by S3 and S5, with S7 showing very low counts. The elevated fecal coliform load resulted from frequent mammal visits to the lake for drinking water, potentially contaminating it with faeces. The increased load is primarily attributed to human activities.
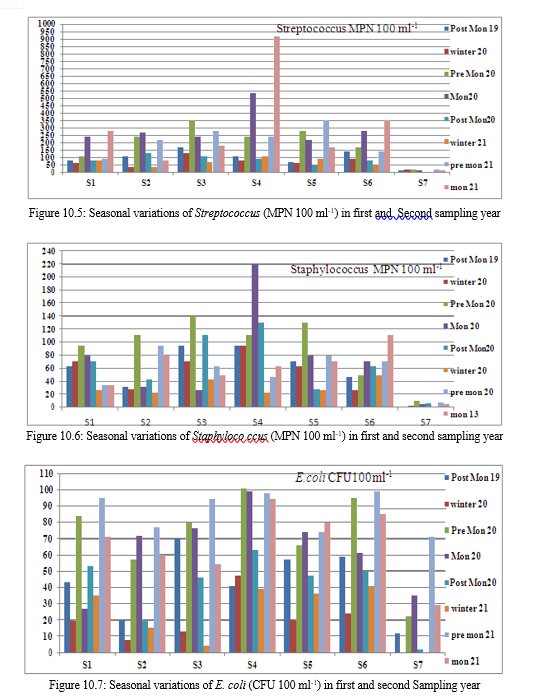
E. Fecal streptococci count
The overall range of fecal streptococci count during the sampling period was 920 MPN 100 ml-1 to 4 MPN 100 ml-1. The count was usually high in monsoon followed by pre monsoon and post monsoon. Lowest count was present in winter in all the samples. Sample S4 had the high counts and S7 had the low counts in almost all the seasons in the sampling period.
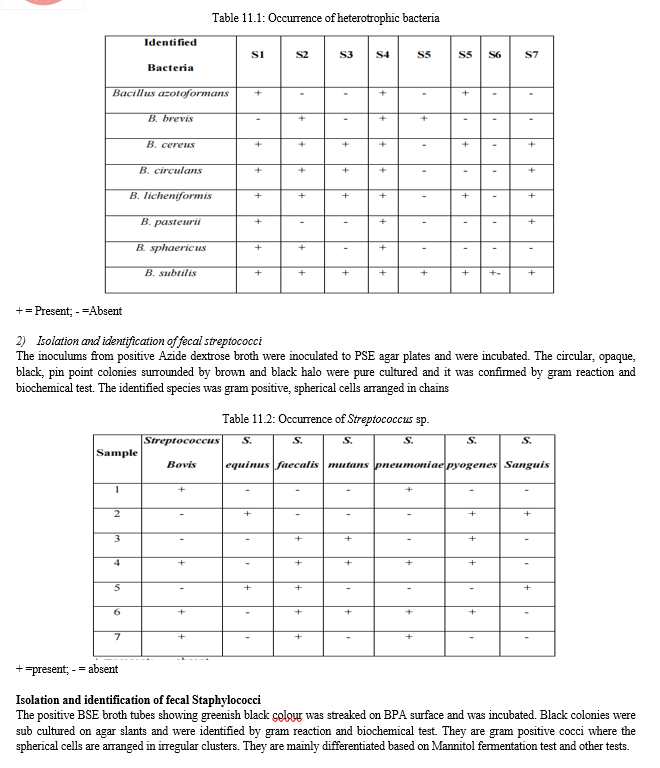
F. Fecal staphylococci count
Range of fecal staphylococci count during the sampling period was 220 MPN 100 ml-1 to 0 MPN 100 ml-1. The count was usually high in S4 followed by S3, S5, S6, S1 and S2. Lowest count was observed in S7 throughout the sampling period. The increase in counts indicates increased pollution and such lake water is not suitable for human activities and may cause skin infections and pneumonia.
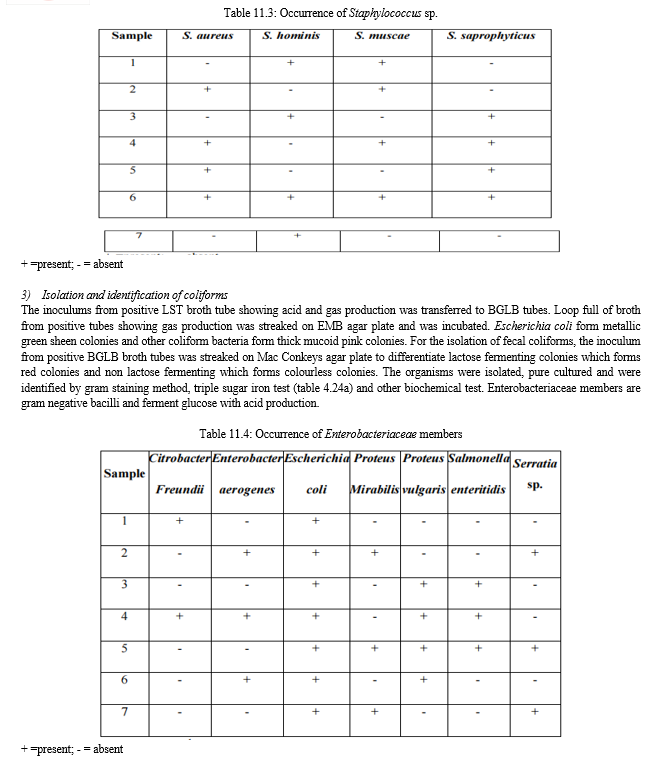
G. Escherichia coli count
The E. coli count was high in pre monsoon and monsoon followed by post monsoon.
It was low in winter. Comparatively, samples S4, S5 and S6 had highest E. coli count.
A count more than 100 will indicate entry of raw sewage and increased pollution. It is one of the widely accepted indicators of fecal pollution and may cause outbreaks of gastrointestinal infection and urinary tract infection.
H. H2S strip test
After inoculation and incubation, appearance of black colour indicates the presence of
H2S producing pathogenic bacteria like species of Salmonella and Citrobacter. The presence of Salmonella sp., Citrobacter sp. and Shigella sp. were confirmed in certain seasons of the sampling period by H2S kit test and triple sugar iron test. The presence of these indicates the presence of other pathogenic bacteria and also increased incidence of water borne infections.
1) Isolation and identification of heterotrophic bacteria
From the result plate some of the isolated colonies were selected, pure cultured and were identified based on morphological characters, gram staining and biochemical tests.
Conclusion
Water is the basic need of all living organism and all have the right of having unpolluted water in nature. Water is a prime natural resource and precious natural asset. Limnology is the study of productivity, structural and physical relationship between the organisms of inland aquatic ecosystem which is in turn regulated by biotic communities. Biodiversity stabilizes human health and ecological balance which is under stress due to increased intervention of humans in ecosystem. Loss of biodiversity will affect the life on earth. Human activities surrounding the catchment area of the lake may release large amount nutrients due to malfunctioning or absent of wastewater treatment, unlawful waste disposal and intensive livestock activities. Regular environmental monitoring may help to reduce harmful effects and help in conservation of water bodies. The bacteria, algae and macrophytes like water Hydrilla verticillata and Pistia strtiotes utilize nitrate and phosphate and reduce their concentration and avoid eutrophication to some extent. Seasonal variation effect the distribution of bacteria and it is usually high in pre monsoon due to convenient temperature and high organic input. Coliforms show irregular seasonal variations. All the bacterial parameters were above the permissible limit of drinking water and of the water used for swimming or recreation. There was the presence of heavy load of heterotrophic bacteria, aerobic bacteria, TC, FC, Streptococcus sp., Staphylococcus sp. and gastrointestinal pathogens like Salmonella sp. and Klebsiella sp. in certain sampling season and this indicates the increase in incidence of water borne infection like diarrhea, dysentery and typhoid and this plays a significant role in public health. Anthropogenic activities affect the microbiological quality of water. Though no single indicator can precisely indicate water quality TC and FC is commonly used as indicators of fecal pollution and HPC is used as indicator of agriculture and industrial waste pollution. As the lake water is used for domestic and agriculture purpose it is mandatory to assess the bacteriological quality periodically to avoid the negative impacts. Regular monitoring for TC and FC is required to prevent water borne diseases. The percentage of death of children under the age of five years in developing countries is high due to water borne disease (WHO, 2004) and this can be avoided to some extent by proper management of water bodies especially those which used as a source of drinking water. Proper treatment of waste and sewage before leaving it into water bodies and educating people regarding the effects of eutrophication may help in conservation of lakes.
References
[1] Adedokun, O. A., Adeyemo, O. K. and Adeleye, E. (2008). Seasonal limnological variation and nutrient load of the river system in Ibadan Metropolis, Nigeria. European Jour. of Scientific Res., 23(1): 98-108. [2] Agnieszka, P. and Walk-Wozniak, E. (2007). Effect of environmental conditions on rotifers and selected phytoplankton species in three sub mountain Dam Southern Polands, Central Europe. Ekologia, 26(2): 132– 142. [3] Ahmad, M. S. (1996). Ecological survey of some algal flora of polluted habitats of Darbhanga. Jour. Environ. Pollut., 3: 147-151. [4] Ahmad, U., Parveen, S., Khan, A. A., Kabir, H. A., Mola, H. R. A. and Ganai, A. H. (2011). Zooplankton population in relation to physico- chemical factors of a sewage fed pond of Aligarh (UP), India. Biology and Medicine, 3(2): 336- 341. [5] Ahmet, T. A., Erdem, C. and Karaytug, S. (2006). Cladocera and Copepoda (crustacea) fauna of Catalan Dam Lake (Adana, Turkey). Jour. of Fisher. and Aqua. Scie., 23(3-4): 427-428. [6] Alikunhi, K.H., S.V. Ganapathi and F. Thivy (1948). Limnology of the Ootacamand lake, Nilgiris II Summer conditions. Proc. 35th Ind. Sci. congress Assoc., Part III, Calcutta, India. [7] Alfred, J.R.B. and M.P.T. Thapa (1996). Limnological investigations on Ward’s lake- A wetland in Shillong, Meghalaya, NE, India. Rec. Zool. Surv. India Occ. paper No. 169, Calcutta, India. [8] Annalakshmi, G. and Amsath, A. (2012). Studies on the hydrobiology of river Cauvery and its Tributaries Arasalar from Kumbakonam region (Tamil Nadu, India) with references to zooplankton. Intern. Jour. of Appl. Biol. and Pharmace. Techn., 3(1): 325-336. [9] APHA (2005). Standard methods for the examination of water and waste water 21st edition, New York, USA.235 [10] Aravind Kumar (1997). Comparative hydrological studies of tropical water bodies with special reference to sewage pollution in south Bihar. Jour. Ecobiol., 9(4): 255-262. [11] Arora, H.C. (1961). Rotifera as indicators of pollution. Bull. CPHERI Nagpur, 3&4: 24-26. [12] Arya, S., Kumar, V., Raikwar, M., Dhaka, A. and Minakshi (2011). Physicochemical analysis of selected surface water samples of Laxmi Tal (Pond) in Jhansi city, U.P, Bundhelkhand region, Central India. Jour.of Experim. Scie. 2 (8): 1-6. [13] Baird, W. (1860). Description of the two new species of Entomostraceous Crustacea from india. Proceeding of the zoological society, london 213– 234. [14] Baker, R. L. (1979). Specific status of Keretella cochearis (Gosse) and K. earliner, Ahlstrom (Rotifera: Brachionidae) Morphological and ecological considerations. Con. Jour. Zool., 57(9), 1719–1722. [15] Baloch, W. A. (1995). Species composition, abundance and seasonal variation of zooplankton in lake Ikeda, Japan. M.S. thesis, university of Kagoshima, Kagoshima. 87-91. [16] Battish, S. K. (1992). Fresh water Zooplankton of India, Oxford and IBH Publication. [17] Berzins, B. and Pejler, B. (1989). Rotifer occurrence in relation to temperature. Hydrobiol. 175: 223- 231. [18] Beyene, H. and Redaie, G. (2011). Assessment of Waste Stabilization Ponds for the Treatment of Hospital Wastewater: The Case of Hawassa University Referral Hospital. World Appl. Scie. Jour. 15 (1): 142-150. [19] Bhagat, P. R. (2008). Study of physico-chemical characteristics of the accumulated water of pond of Lohara, at Yavatmal (MS) India. Rasayan Jour. of chem. 1(1):195-197. [20] Bhatt, L. R., Lacoul, P., Lekhak, H. D. and Jha, P. K. (1999). Physico chemical characteristics and phytoplanktons of Taudaha Lake, Kathmandu, Poll. Res. 18 (4): 353-358
Copyright
Copyright © 2024 Dr. Pooja Narwat. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET61110
Publish Date : 2024-04-27
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

